Organic dye centered on phenanthrene and carbazole, preparation method thereof and organic dye sensitized solar cell
An organic dye, phenanthrocarbazole technology, applied in the field of organic dye-sensitized solar cells, can solve the problems of specific efficiency gap, limit the application of noble metal dyes, and limited reserves of precious metals, and achieve low cost, environmental friendliness, and avoid the use of heavy metals Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
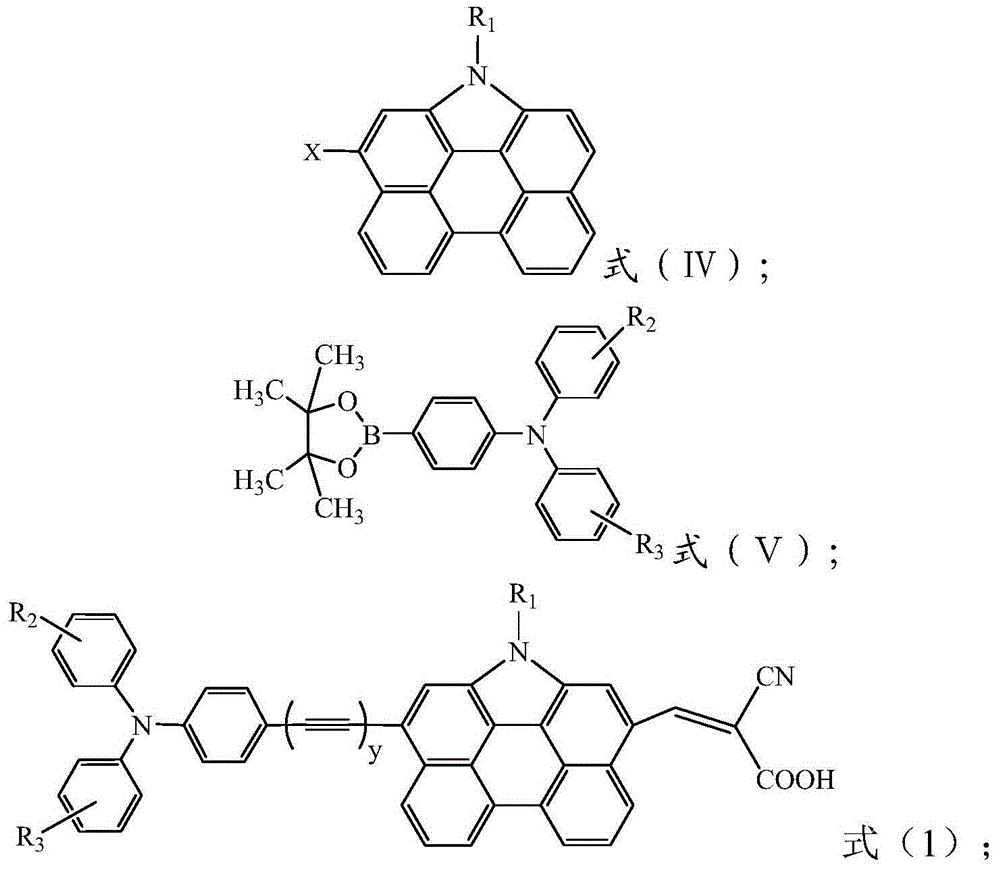
[0054] The present invention also provides a method for preparing an organic dye with phenanthrocarbazole as the core, comprising:
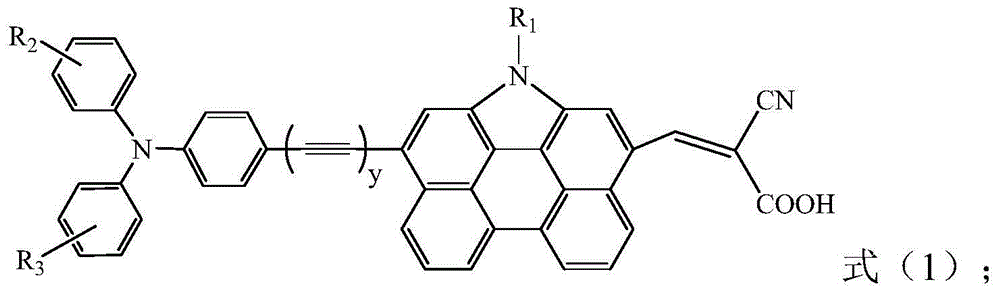
[0055] The compound shown in formula (IV) is reacted with N,N-dimethylformamide and phosphorus oxychloride, and then with the triphenylamine borate derivative shown in formula (Ⅴ), in palladium acetate, Sphos and potassium phosphate Under the effect of reacting, finally react with cyanoacrylic acid under the effect of ammonium acetate, obtain the organic dye shown in formula (1);
[0056]
[0057] in,
[0058] R 1 from C 1 ~C 18 alkyl;
[0059] R 2 , R 3 Independently selected from H, C 1 ~C 18 Alkyl or C 1 ~C 18 Any of the alkoxy groups;
[0060] y is 0;
[0061] x is halogen.
[0062] First, the compound shown in formula (IV) is reacted with N,N-dimethylformamide and phosphorus oxychloride, and the reaction scheme is as follows:
[0063]
[0064] Specifically, first mix the compound represented by formula (IV) with 1,2-dichl...
Embodiment 1
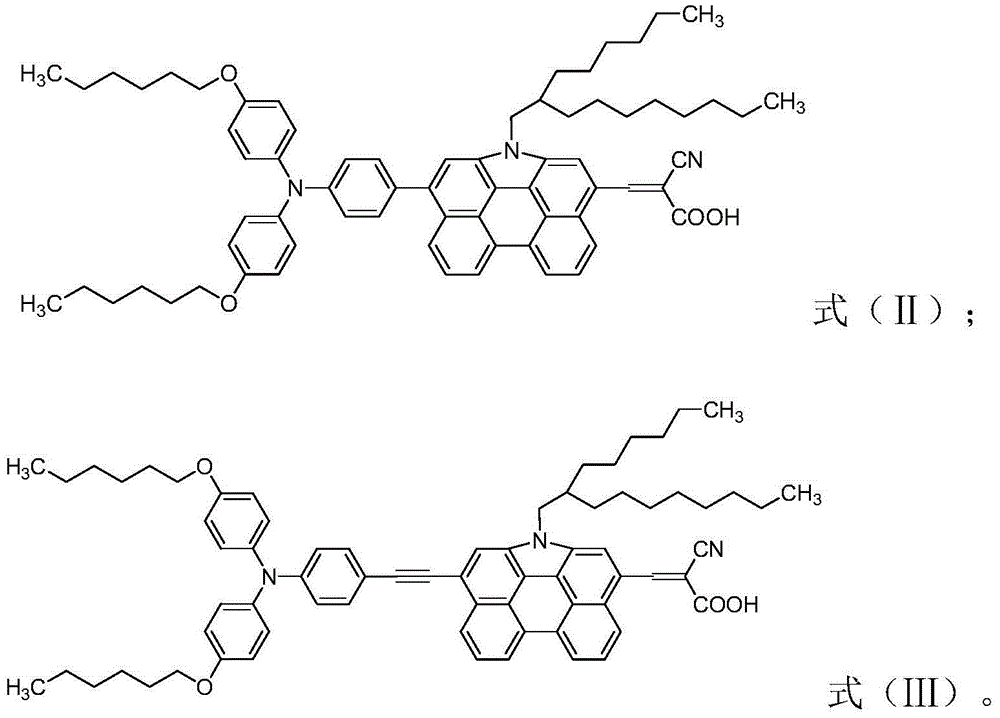
[0105] The preparation of the organic dye of formula (II) structure, synthetic route is as follows:
[0106]
[0107] Compound 1 was synthesized according to references (W. Jiang, H. Qian, Y. Li, Z. Wang, J. Org. Chem. 2008, 73, 7369.).
[0108] In a dry Schlenk reaction flask, dissolve 2.00g of compound 1 in 20mL of 1,2-dichloroethane, cool the reaction system to 0°C on ice, and add 0.31mL of N,N-dimethylformamide to the reaction system And 0.40mL of phosphorus oxychloride, heated up to 40 ℃ and reacted overnight.
[0109] After the reaction, cool the reaction system to room temperature, add 20 mL of water and stir for 2 hours, extract three times with chloroform, combine the organic phases, dry the organic phases with anhydrous sodium sulfate, filter to remove the desiccant, concentrate the filtrate, and use ethyl acetate / petroleum ether (Volume ratio 1 / 20) was used as a developing solvent for column chromatography to obtain 1.76 g of intermediate 2 with a yield of 84%. ...
Embodiment 2
[0134] Preparation of organic dye Ⅲ
[0135] The reaction scheme is as follows:
[0136]
[0137] Synthesis of intermediate 5:
[0138] Compound 4 was synthesized according to literature (R. Li, J. Liu, N. Cai, M. Zhang and P. Wang, J. Phys. Chem. B, 2010, 114, 4461.).
[0139] In a dry Schlenk reaction flask, 500mg of compound 4 and 218mg of triisopropylsilylacetylene were dissolved in 20ml of toluene, and 67mg of Pd(PPh 3 ) 2 Cl 2 , 18mg cuprous iodide and 2mL triethylamine. The reaction system was heated to reflux overnight.
[0140] After the reaction was complete, the temperature of the system was lowered to room temperature. After adding 30 mL of distilled water, extracted three times with chloroform, combined the organic phases, dried the organic phases with anhydrous sodium sulfate, filtered to remove the desiccant, concentrated the filtrate, and used ethyl acetate / petroleum ether (volume ratio 1 / 50) was used as a developing agent for column chromatography to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com