A kind of double-layer osmotic pump controlled-release tablet containing melatonin and its preparation method
An osmotic pump controlled release, melatonin technology, applied in the directions of medical preparations containing active ingredients, medical preparations with non-active ingredients, pharmaceutical formulas, etc. Drug release rate interference and other problems to achieve the effect of enhancing immunity, ensuring sleep time, and high utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
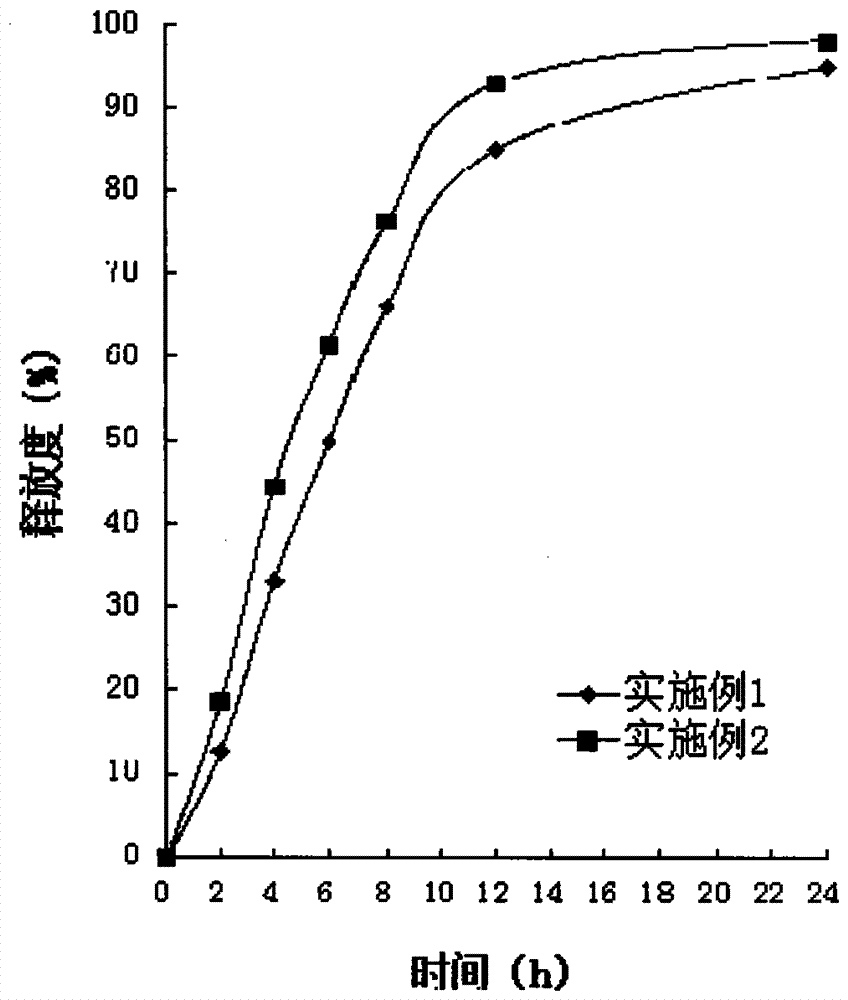
Examples
Embodiment 1
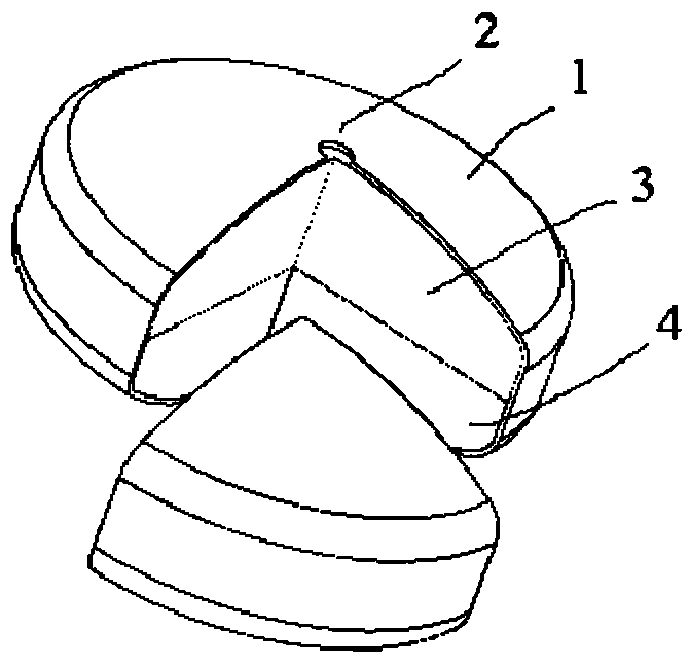
[0026] Such as figure 1 As shown, the osmotic pump controlled-release tablet containing melatonin described in the embodiment of the present invention is composed of an osmotic pump tablet and a moisture-proof protective film on its outer layer, wherein the osmotic pump tablet includes a double-layer tablet core and a coating half. Permeable membrane 1, the outer surface of the double-layer tablet core is wrapped with a semipermeable membrane 1 and a drug release hole 2 is added on the semipermeable membrane 1, the upper layer of the double-layer tablet core is a melatonin-containing drug layer 3, The lower layer is the push layer 4 containing osmotic pressure active substances. When the controlled-release tablet enters the gastric juice, the moisture-proof protective film on the outer layer disintegrates rapidly, and the melatonin in the protective film is completely released, providing the human body with the first burst dose. Then, The water molecules in the digestive tract...
Embodiment 2
[0036] Such as figure 1 As shown, the osmotic pump controlled-release tablet containing melatonin described in the embodiments of the present invention, the controlled-release tablet formula is as follows:
[0037] ①Based on the total dosage per 1000 tablets, the melatonin-containing drug layer 3 is composed of the following materials:
[0038] Melatonin 3g, sodium chloride 60g, mannitol 30g, lactose 30g, dextrin 10g, polyethylene oxide (molecular weight 300,000, PEO) 1.0g, polyvinylpyrrolidone (PVP K29 / 32) 6g, stearic acid Magnesium 1.4g and appropriate amount of ethanol;
[0039] ②Based on the total dosage per 1000 tablets, the push layer 4 containing osmotic pressure active substances is composed of the following materials:
[0040] Sodium chloride 40g, lactose 15g, polyethylene oxide (molecular weight: 5 million, PEO) 40g, polyvinylpyrrolidone (PVPK29 / 32) 9.5g, iron oxide red 1g, magnesium stearate 1g and ethanol amount;
[0041] ③Based on the total dosage per 1000 piec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com