Method for degrading rhodamine B and indigo organic pollutant
A technology of organic pollutants and indigo, applied in the field of photocatalytic materials, can solve problems such as application limitations, and achieve the effects of simple synthesis method, good catalytic degradation effect, and low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1.1. Preparation of degradation catalyst
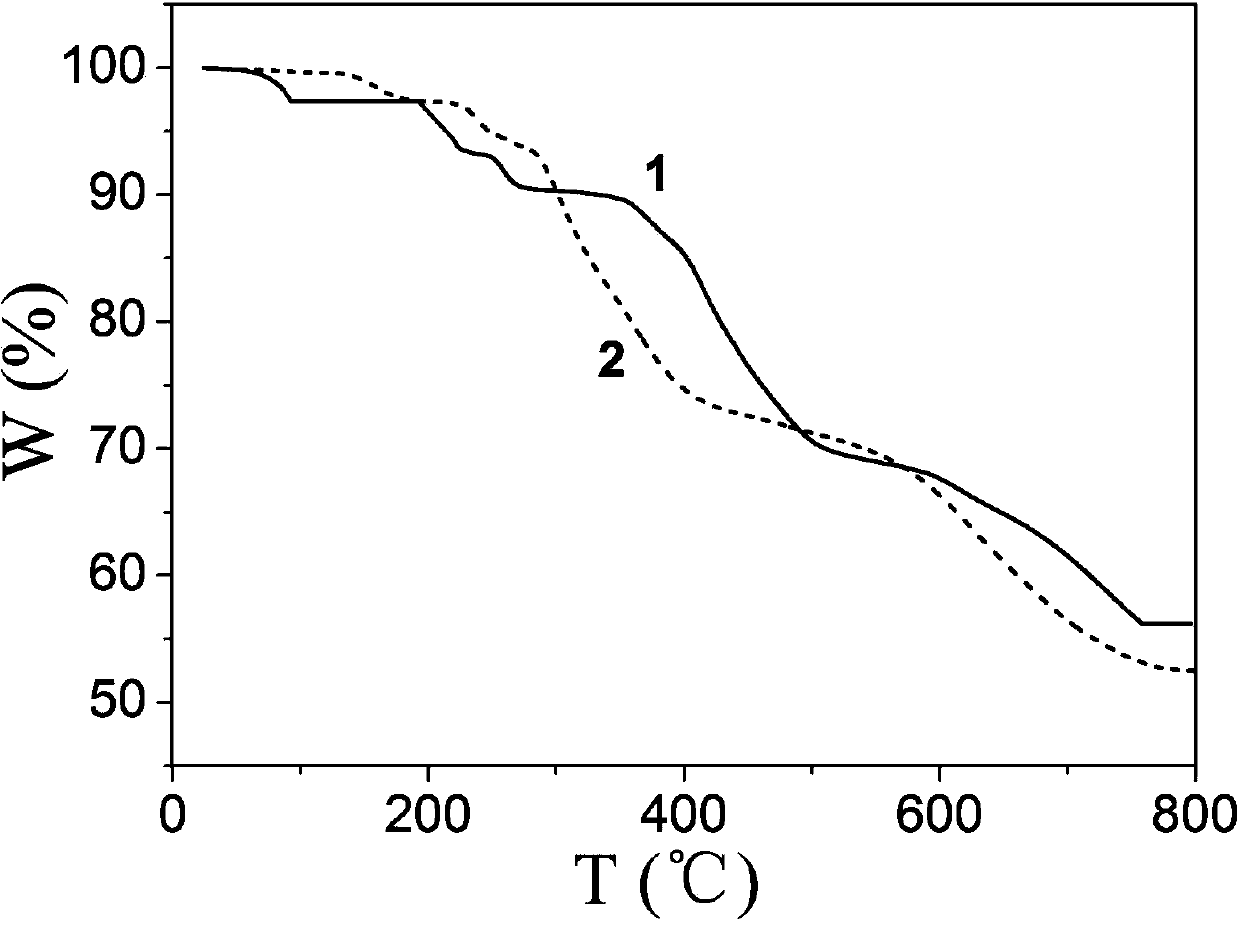
[0045] 0.1mmol CuCl 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarbonyl)-1,2-ethane, 0.10mmolNa 3 [CrMo 6 h 6 o 24 ]·8H 2 O (sodium chromium molybdate octahydrate) and 12.0 mL of deionized water were successively added to a 25 mL beaker, stirred at room temperature for 30 min to obtain a suspension mixture, and the pH of the suspension mixture was adjusted to 1.8 with 1.0 mol / L HCl solution, and then transferred to In a 25mL autoclave, heat up to 110°C at a heating rate of 15°C / h, keep the temperature under hydrothermal conditions for 48 hours, and then cool down to room temperature at a cooling rate of 2.5°C / h to obtain dark green blocky crystals. Rinse twice with water and absolute ethanol alternately, and dry naturally at room temperature to obtain the complex H{CuL 1 0.5 [CrMo 6 (OH) 6 o 18 ](H 2 O)} 0.5L 1 , where L 1 for N , N '-Bis(3-pyridinecarbonyl)-1,2-ethane, the structural formula is: , the product...
Embodiment 2
[0050] 1.1. Preparation of degradation catalyst
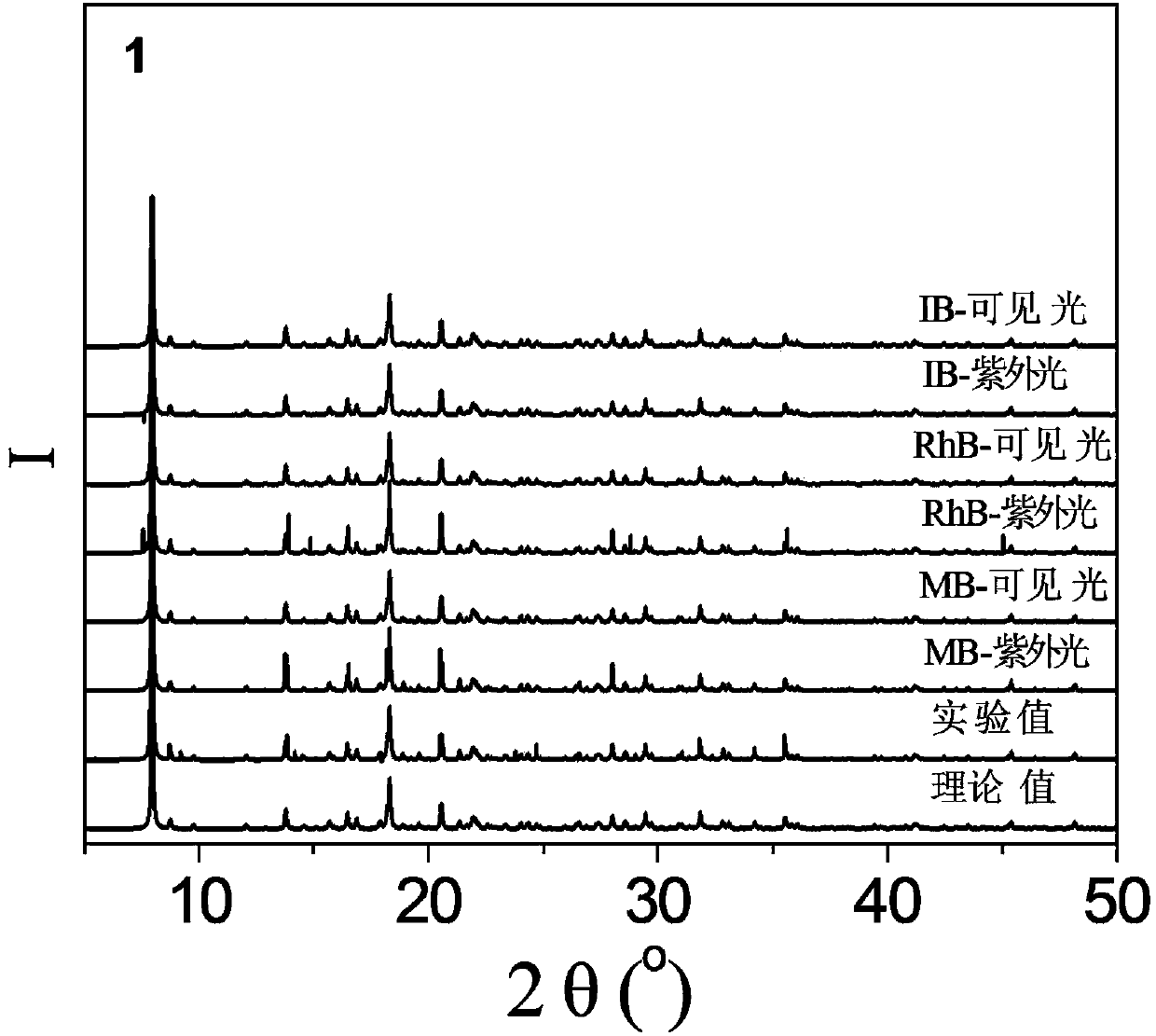
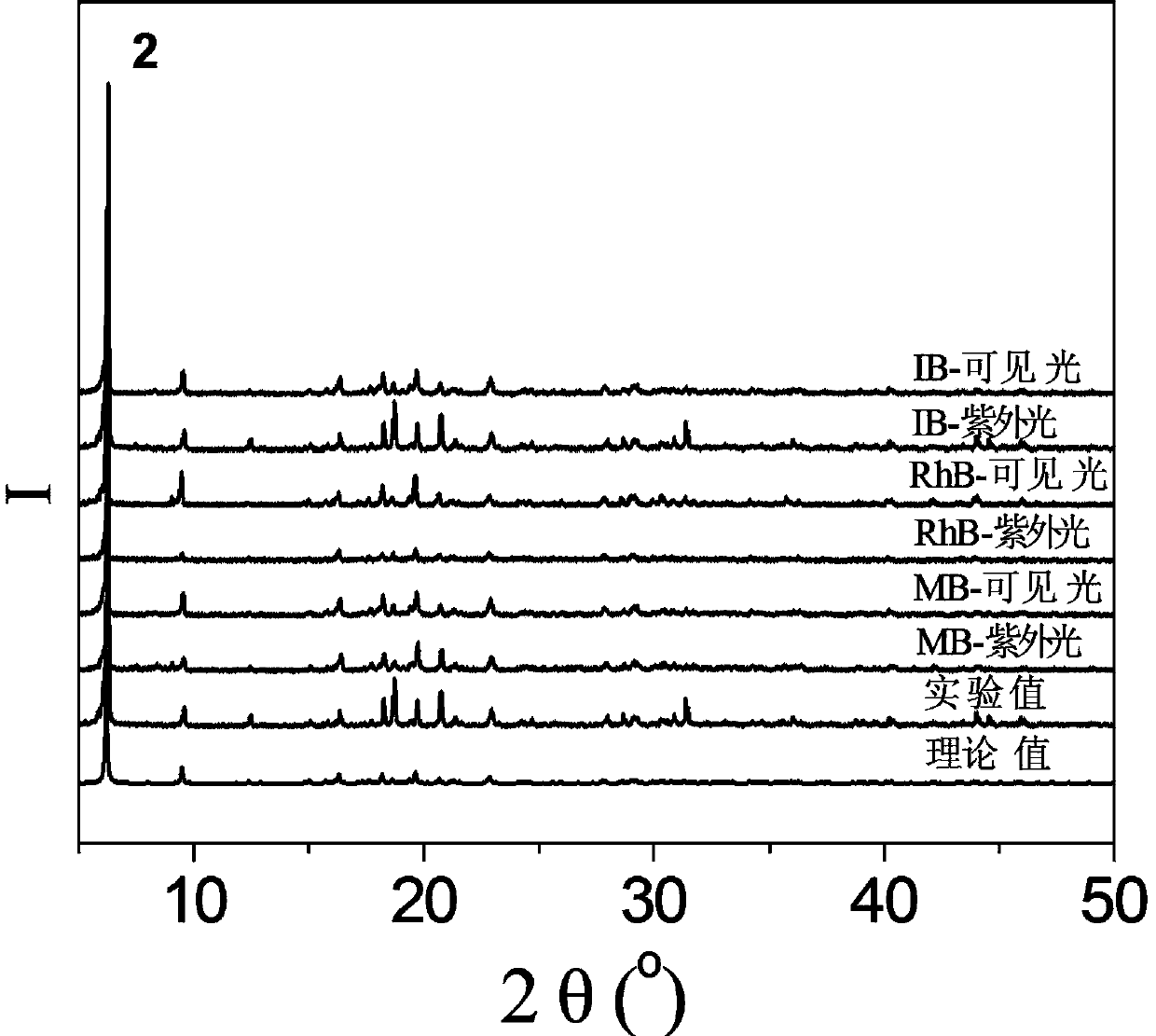
[0051] 0.40mmol CuCl 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarbonyl)-1,2-ethane, 0.30mmol Na 3 [CrMo 6 h 6 o 24 ]·8H 2 0 and 7.0mL of deionized water were added to the 25mL beaker successively, and stirred at room temperature for 10min to obtain a suspension mixture. After the pH of the suspension mixture was adjusted to 2.5 with 0.5mol / L HCl solution, it was transferred to a 25mL autoclave. Raise the temperature to 130°C at a heating rate of 5°C / h, keep it warm for 120h under hydrothermal conditions, and cool down to room temperature at a cooling rate of 5°C / h to obtain dark green blocky crystals, which were washed alternately with deionized water and absolute ethanol for 3 times, air-dried at room temperature to obtain the complex H{CuL 1 0.5 [CrMo 6 (OH) 6 o 18 ](H 2 O)} 0.5L 1 , where L 1 for N , N '-bis(3-pyridinecarbonyl)-1,2-ethane, the productive rate is 28%, and its PXRD diffraction pattern is as f...
Embodiment 3
[0056] 1.1. Preparation of degradation catalyst
[0057] 0.5mmol CuCl 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarbonyl)-1,2-ethane, 0.24mmol Na 3 [CrMo 6 h 6 o 24 ]·8H 2 0 and 11.0mL of deionized water were added to the 25mL beaker successively, and stirred at room temperature for 30min to obtain a suspension mixture. After the pH of the suspension mixture was adjusted to 2.1 with 1.0mol / L HCl solution, it was transferred to a 25mL autoclave. Raise the temperature to 120 °C at a heating rate of 10 °C / h, keep it under hydrothermal conditions for 96 h, and cool down to room temperature at a cooling rate of 10 °C / h to obtain dark green blocky crystals, which were washed alternately with deionized water and absolute ethanol for 2 times, air-dried at room temperature to obtain the complex H{CuL 1 0.5 [CrMo 6 (OH) 6 o 18 ](H 2 O)} 0.5L 1 , L 1 for N , N '-bis(3-pyridinecarbonyl)-1,2-ethane, the productive rate is 34%, and its PXRD diffraction pattern is as follows...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com