Fluorescein hydrazide derivatives, preparation method of fluorescein hydrazide derivatives, modified TiO2 functional material, preparation method of modified TiO2 functional material, and sensor
A technology of fluorescein hydrazide and functional material is applied to modified TiO2 functional material, preparation method and sensor prepared by using the functional material, fluorescein hydrazide derivative and the field of preparation thereof, which can solve the problem of inconvenience of carrying and slow detection speed. , detection accuracy and low sensitivity, etc., to ensure the effect of combining performance and easy connection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The fluorescein hydrazide derivative of this embodiment has a structural formula as shown in formula 5:
[0082]
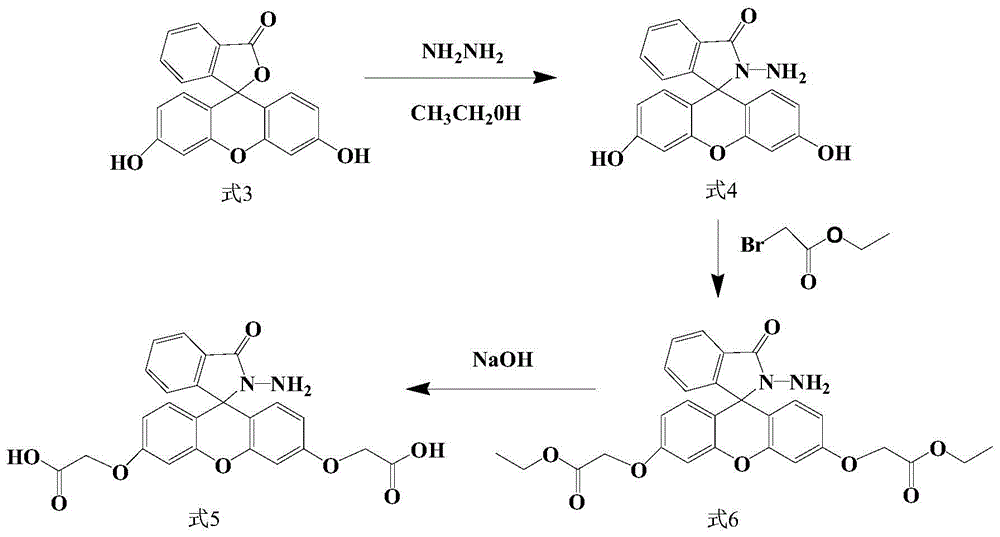
[0083] The preparation method of the fluorescein hydrazide derivative of the present embodiment comprises the following steps (the synthetic route is as follows: figure 1 shown):
[0084] 1) Measure 20ml of absolute ethanol and place it in a three-necked flask, weigh 2g of fluorescein (the structural formula is shown in formula 3) into the three-necked flask, adjust the temperature to 110°C and reflux to dissolve the fluorescein. After the pigment dissolves, slowly add hydrazine hydrate, the molar ratio of fluorescein to hydrazine hydrate is 1:30, then magnetically stir and reflux for 3 hours, use thin-layer chromatography to detect whether the reaction is complete, and when the reaction is complete, recrystallize and purify to obtain fluorescein Hydrazide (structural formula shown in formula 4);
[0085]2) Take 0.6g of fluorescein hydrazide and potass...
Embodiment 2
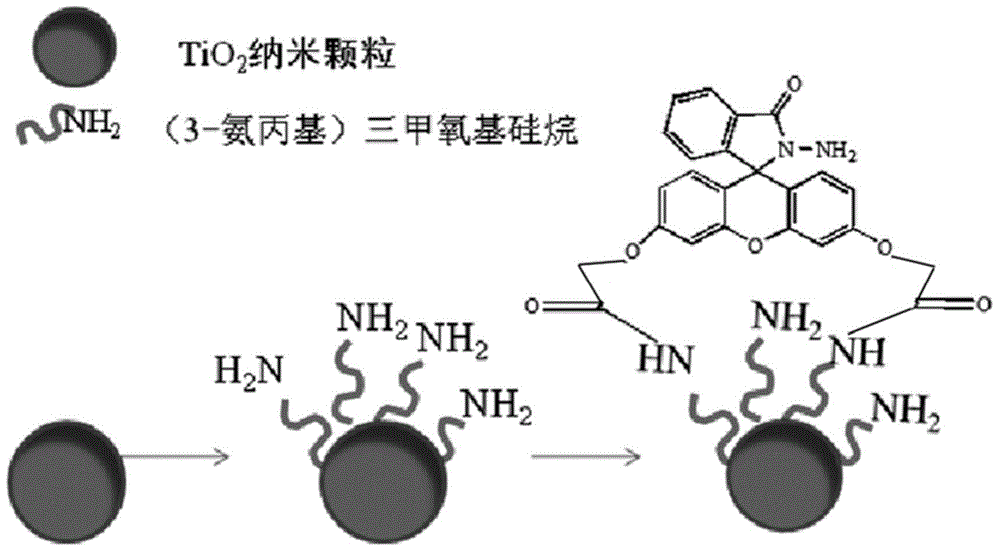
[0088] The modified TiO of this embodiment 2 Functional materials, including TiO 2 nanoparticles, the TiO 2 A group A-1 with the structural formula shown in Formula 7 is attached to the surface of the nanoparticle:
[0089]
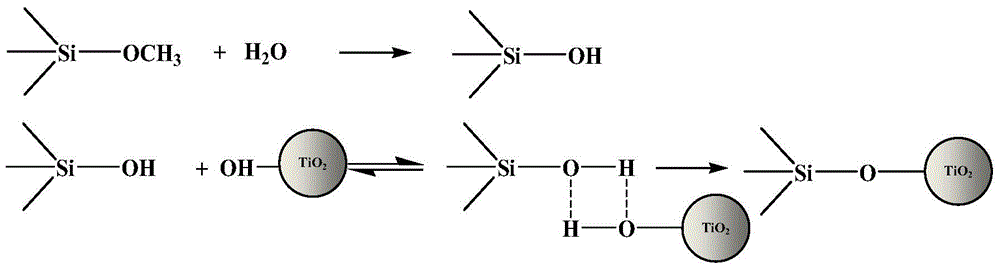
[0090] The compound A-1 is prepared by reacting 3-aminopropyltrimethoxysilane with the fluorescein hydrazide derivative obtained in Example 1. The terminal amino group of 3-aminopropyltrimethoxysilane condenses with the carboxyl group of the fluorescein hydrazide derivative obtained in Example 1 to form an amide bond.
[0091] The modified TiO of this embodiment 2 The preparation method of functional material comprises the following steps (synthetic route such as figure 2 shown):
[0092] a) 1g of TiO 2 Disperse the nanoparticles in 5ml of dichloromethane, then add 0.3ml of 3-aminopropyltrimethoxysilane, stir, and at the same time heat from room temperature to 50°C within 90min, keep stirring for 30min, and then distill off the dichloride under ...
Embodiment 3
[0104] The sensor of the present embodiment is that the fluorescein hydrazide derivative obtained in embodiment 2 is modified TiO 2 Functional materials (nanoparticles) were spin-coated onto gold electrodes.
[0105] The sensor of this embodiment detects Cu 2+ The application of aspect, specifically: adopt described sensor, detect Cu with AC impedance method 2+ .
[0106] Fluorescein hydrazide derivative modified TiO obtained in embodiment 2 2 Functional materials combined with Cu 2+ The schematic diagram of the reaction principle is as Figure 6 shown.
[0107] The sensor obtained in this embodiment detects Cu 2+ effect is tested. The test content includes electrochemical signal test, minimum detection limit test and selectivity test.
[0108] The instrument used for the electrochemical test is an electrochemical workstation 660D (Shanghai Chenhua), the test method is AC impedance method (EIS), and the electrolyte is 5mM K 3 [Fe(CN) 6 ] / K 4 [Fe(CN) 6 ] (1:1) in PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com