Application of dioscin to preparation of renal injury protection medicament
A technology of diosgenin and kidney injury, which is applied in the application field of diosgenin in the preparation of kidney injury protection drugs, and can solve the problems of no diosgenin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
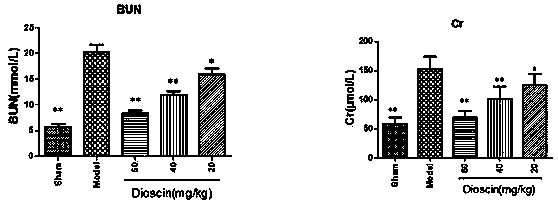
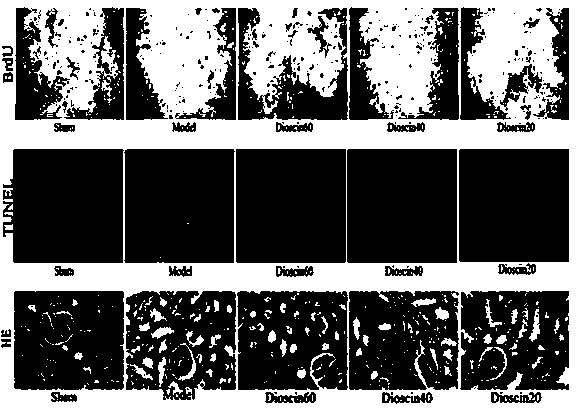
[0023] Example 1 Study on the preventive effect of diosgenin on acute kidney injury in rats with renal ischemia-reperfusion
[0024] 1. Experimental method
[0025] 1.1 Animal model establishment and grouping
[0026] 50 healthy adult SD male rats at 8-10 weeks were randomly divided into 5 groups: sham operation group (Sham), ischemia-reperfusion group (Model), high-dose administration group (dioscin 60 mg / kg), medium-dose Administration group (diosgenin 40 mg / kg), low dose administration group (diosgenin 20 mg / kg). The administration group underwent surgery 7 days after the administration.
[0027] The experimental animals were fasted for 12 h before the operation, had free access to water, and were anesthetized by intraperitoneal injection of 10% chloral hydrate solution (3.5 mL / kg) before the operation, and fixed on the operating table. Make an incision in the middle of the abdomen along the midline of the abdomen, cut and separate the skin, muscles, and linea alba layer...
Embodiment 2
[0060] Example 2 Study on the therapeutic effect of diosgenin on renal fibrosis in rats caused by unilateral ureteral ligation
[0061] 1. Experimental method
[0062] 1.1 Animal model establishment and grouping
[0063] Male SD rats were randomly divided into 5 groups, 10 in each group: sham operation group (Sham group), unilateral ureteral ligation group (Model group), high-dose administration group (diosgenin 60 mg / kg), middle-dose administration group group (diosgenin 40 mg / kg), low-dose administration group (diosgenin 20 mg / kg).
[0064] The experimental animals were fasted for 12 h before operation and had free access to water, and were anesthetized by intraperitoneal injection of 10% chloral hydrate solution (3.5 mL / kg) before operation. Model group and drug treatment group: the left abdominal incision was made to open the abdominal cavity, the left ureter was bluntly separated, the upper middle 1 / 3 was double ligated with 4-0 suture, and the abdominal cavity was clos...
Embodiment 3
[0087] Example 3 Study on the therapeutic effect of diosgenin on renal tubular epithelial cell hypoxia / reoxygenation injury model
[0088] 1. Experimental method
[0089] 1.1 Recovery, culture, subculture and experimental grouping of NRK-52E cells
[0090] The frozen NRK-52E cells were taken out from the liquid nitrogen tank and thawed in a water bath at 37°C. Aspirate the cell suspension and add DMEM / F12 (10% FBS) culture medium, centrifuge at 1000 r / min for 5 min, discard the supernatant and repeat washing once. Dilute and mix with DMEM / F12 (10% FBS) culture medium, inoculate in 50 ml culture flask, place in 5% CO at 37°C 2The cells were left standing in the incubator for 12 hours, and the medium was changed to remove non-adherent cells. After the cells were over 80% full, they were digested and passaged with 0.25% trypsin. The experiment was randomly divided into 5 groups, namely the blank group, the model group, and the three dosage administration groups. Each group was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com