A kind of preparation method of ziprasidone key intermediate
A technology for ziprasidone and intermediates, which is applied in the field of chemical pharmaceuticals, and can solve problems such as difficult industrial implementation, complicated processing, and reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
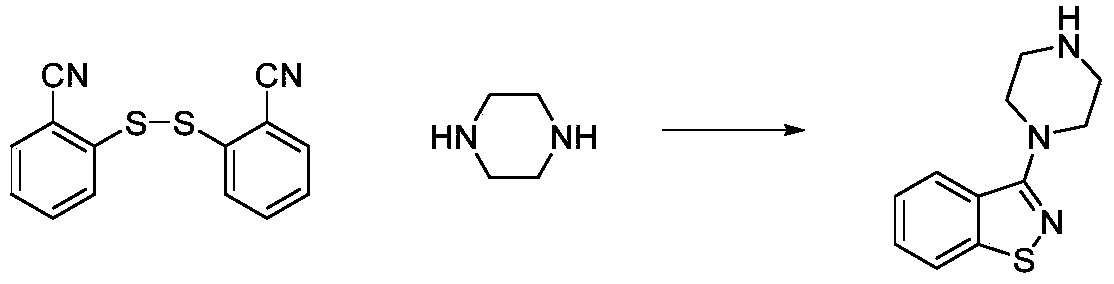
Method used
Image
Examples
Embodiment 1
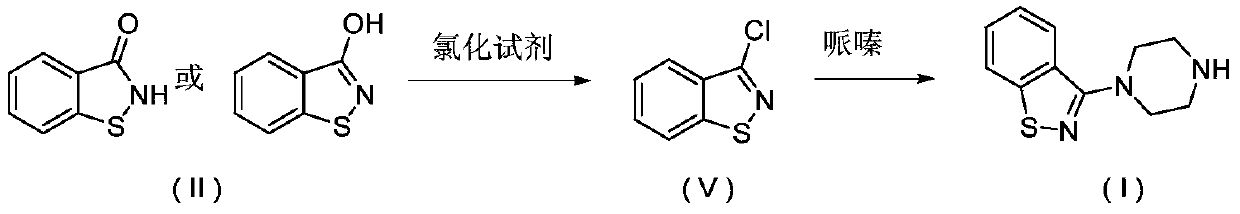
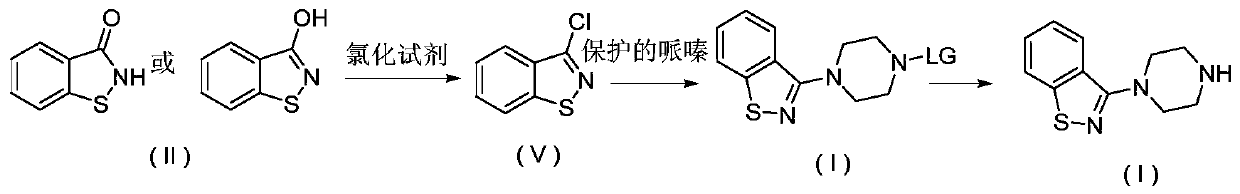
[0106] (1) Preparation of benzo[d]isothiazole-3-trifluoromethanesulfonate:
[0107] Add 1.5g of the compound of formula II, 5mL of dichloromethane, and 1.5g of triethylamine into the reaction kettle, cool down to 5-10°C, add 3g of trifluoromethanesulfonic anhydride dropwise, keep warm until the reaction is complete, and add 5g water, extraction, and liquid separation to obtain a dichloromethane solution of benzo[d]isothiazole-3-trifluoromethanesulfonate, and proceed to the next reaction directly.
[0108] (2) Preparation of 3-(1-piperazinyl)-1,2-benzisothiazole formula I compound:
[0109] Dissolve 2.5g of anhydrous piperazine into 5ml of dichloromethane, control the temperature at 15-20°C, and add dropwise the dichloromethane solution of benzo[d]isothiazole-3-trifluoromethanesulfonate obtained in the previous step , temperature controlled until the reaction was complete, filtered, washed with water, separated, and the solvent was recovered from the organic layer under reduce...
Embodiment 2
[0112] (1) Preparation of benzo[d]isothiazole-3-methanesulfonate:
[0113] Add 1.5kg of compound of formula II, 5L of dichloromethane, and 1.5kg of pyridine into the reaction kettle, cool down to 5-10°C, add 1.43kg of methanesulfonic acid chloride dropwise, keep warm until the reaction is complete after the dropwise addition, add 5kg of 5% sulfuric acid Aqueous solution, extraction, liquid separation, organic layer recovered solvent to obtain benzo[d]isothiazole-3-methanesulfonate 2.06kg, yield 90.1%, purity 97.5%.
[0114] (2) Preparation of 3-(1-piperazinyl)-1,2-benzisothiazole formula I compound hydrochloride:
[0115] Dissolve 1.7kg of anhydrous piperazine into 5L of ethanol, raise the temperature to 50-60°C, add 2kg of benzo[d]isothiazole-3-methanesulfonate in batches, control the temperature until the reaction is complete, filter, and pass into Hydrogen chloride to saturation, filter, collect filter cake and dry, recrystallize 2.04kg of 3-(1-piperazinyl)-1,2-benzisothia...
Embodiment 3
[0117] (1) Preparation of benzo[d]isothiazole-3-p-toluenesulfonate:
[0118] Add 1.5kg of the compound of formula II and 2kg of 4-methylpyridine into the reaction kettle, cool down to 5-10°C, add 2.43kg of p-toluenesulfonic acid chloride in batches, naturally warm up to room temperature after the dropwise addition, and stir until the reaction is complete , added to 15kg of water to precipitate a solid, the filtrate recovered 4-picoline, and dried the solid to obtain 2.76kg of benzo[d]isothiazole-3-p-toluenesulfonate, with a yield of 90% and a purity of 98.5%.
[0119] (2) Preparation of 3-(1-piperazinyl)-1,2-benzisothiazole formula I compound hydrochloride:
[0120] Dissolve 1.94kg of piperazine hexahydrate into 5L of water, raise the temperature to 60-70°C, add 1.5kg of benzo[d]isothiazole-3-p-toluenesulfonate in batches, control the temperature until the reaction is complete, and then lower the temperature to 15 -20°C, add ethyl acetate for extraction, pass through the orga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com