T lymphocyte culture medium without any animal original or human original component and preparation method thereof
A technology of lymphocytes and culture medium, applied in the field of culture medium formulation and preparation of human T lymphocytes, can solve problems such as potential safety hazards, and achieve the effects of high growth rate, high cell density and good culture effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the configuration of culture medium
[0037] Culture medium configuration method
[0038] Step 1, configuring solution 1: Weigh 2 mg of cholesterol, 2 mg of oleic acid, and 2 mg of linoleic acid and dissolve them in 1 ml of absolute ethanol.
[0039] Step 2, configuring basal medium: mixing IMDM medium and DMEM / F12 medium at a ratio of 1:1.
[0040] Step 3: Add 3 g of recombinant human serum albumin, 50 mg of recombinant human transferrin, 10 mg of recombinant human insulin, 1 ml of solution one, 2 mg of ethanolamine, 0.001 mg of copper sulfate, 0.5 mg of zinc sulfate, and Manganese chloride 0.0005mg, gentamicin 10000IU, stir to fully dissolve, adjust the pH value between 6.8-7.2 with 0.1mol / L hydrochloric acid and 0.1mol / L sodium hydroxide, 0.1um filter membrane filter to sterilize, pack in aliquots , 4-8 degrees to save.
[0041] Step 4, preparation of heat-inactivated autologous plasma. Place the aseptically preserved plasma in a water bath at 56 de...
Embodiment 2
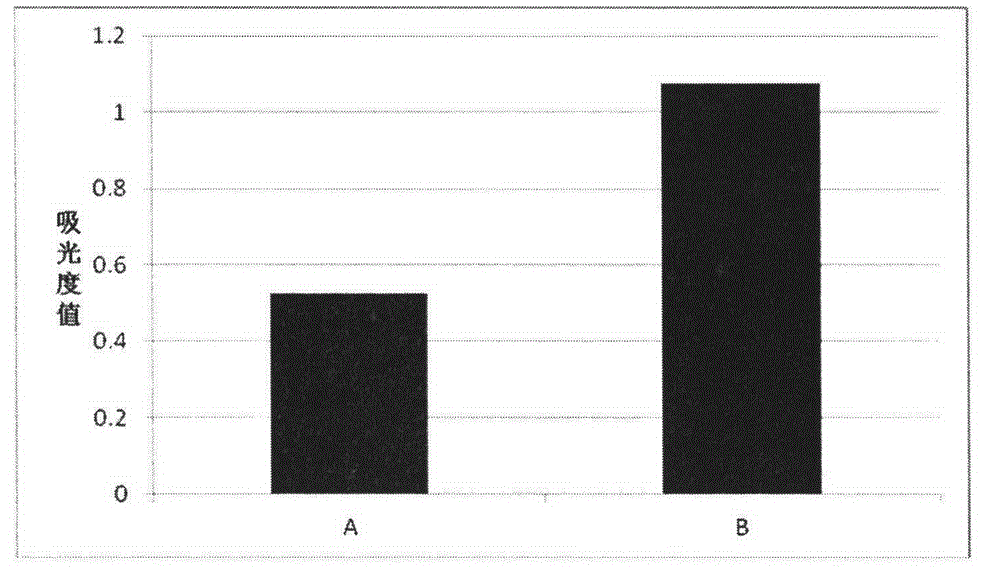
[0043] Example 2: Effect of Autologous Plasma on Lymphocyte Proliferation Activity
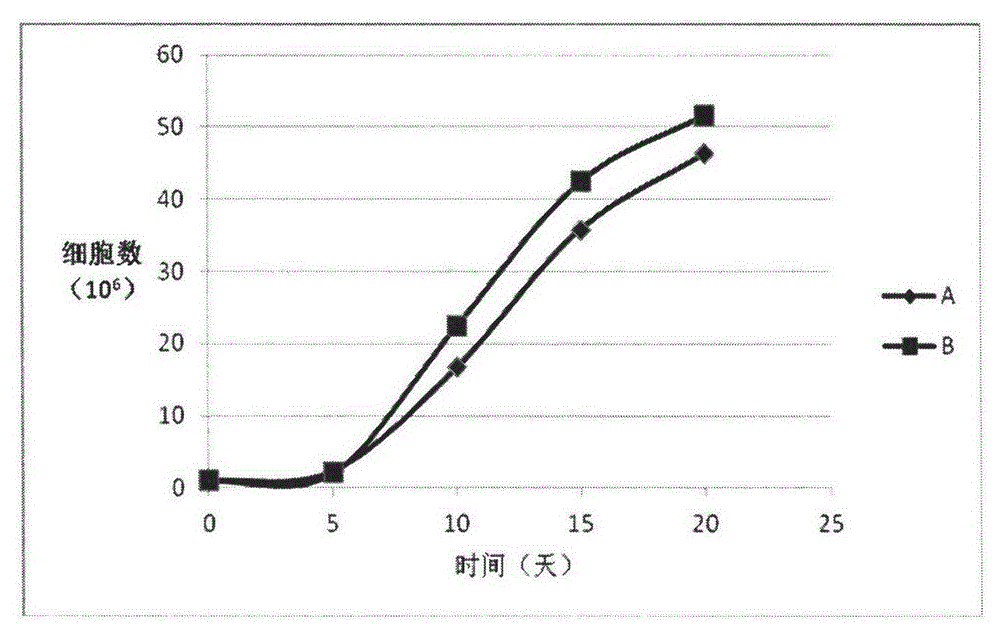
[0044] Heparin anticoagulation collected 50ml of peripheral blood, centrifuged at 3000 rpm for 10 minutes, and separated plasma and cells. The plasma was collected into a 15ml centrifuge tube, inactivated in a 56-degree water bath for 30 minutes, centrifuged to remove the precipitate, and the supernatant was collected for later use. The cells were diluted to the original volume with physiological saline containing 1% human albumin, added to the lymphocyte separation medium, and the peripheral blood mononuclear cells were separated by density gradient centrifugation. Collect the cells at the interface, add physiological saline containing 1% human serum albumin and centrifuge at 1000 rpm for 10 minutes, and remove the supernatant. Set as two groups, the culture medium used in the first group is the culture medium prepared in Step 2 of Example 1, the culture medium used in the second group is th...
Embodiment 3
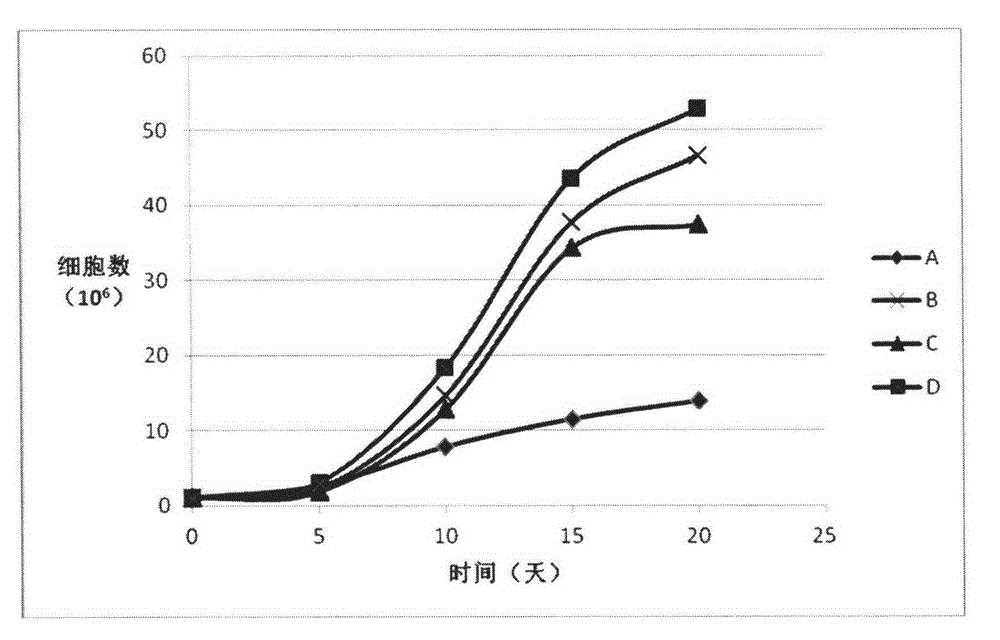
[0046] Embodiment 3: Comparative experiment of human serum albumin and recombinant human serum albumin
[0047] Heparin anticoagulation collected 50ml of peripheral blood, centrifuged at 3000 rpm for 10 minutes, and separated plasma and cells. The plasma was collected into a 15ml centrifuge tube, inactivated in a water bath at 56°C for 30 minutes, centrifuged to remove the precipitate, and the supernatant was collected to obtain heat-inactivated autologous plasma, which was stored at 4°C for later use. The cells were diluted to the original volume with physiological saline containing 1% human albumin, added to the lymphocyte separation medium, and the peripheral blood mononuclear cells were separated by density gradient centrifugation. Collect the cells at the interface, add physiological saline containing 1% human serum albumin and centrifuge at 1000 rpm for 10 minutes, and remove the supernatant. Add 1% heat-inactivated autologous plasma to the medium to make a complete med...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com