Organic solid catalyst and preparation method thereof
A technology of organic solids and catalysts, applied in organic chemistry, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of limited synthesis and use of acidic catalysts, low stability of HMF, and preparation process Complicated and other issues, to achieve the effect of short time, simple preparation process and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of organic solid catalyst
[0030] Add 1g of divinylbenzene to 128g of cyclohexane solvent, stir for 20-30min to make it evenly mixed, drop 0.12g of boron trifluoride ethyl ether (BFEE) into the mixed system, and react at 10°C for 30s , adding 2g of methanol to terminate the reaction; the obtained product was washed with methanol or ethanol and water for 3 to 5 times, and dried under vacuum at 50 to 55°C for 20 to 24 hours to obtain a nanotubular polymer with hydrophobic properties;
[0031] Take 0.25g of the material obtained above, grind it into powder, add it to 50mL of 98% concentrated sulfuric acid solution, stir at 10°C for 2h, and dry it in vacuum at 70-80°C to obtain a nanotube-shaped hydrophobic acidic catalyst.
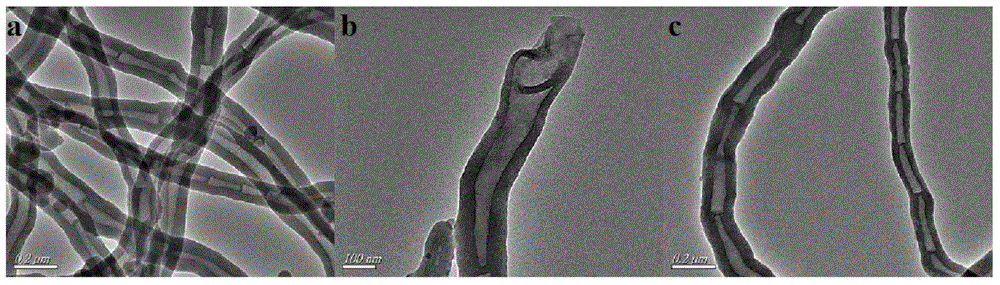
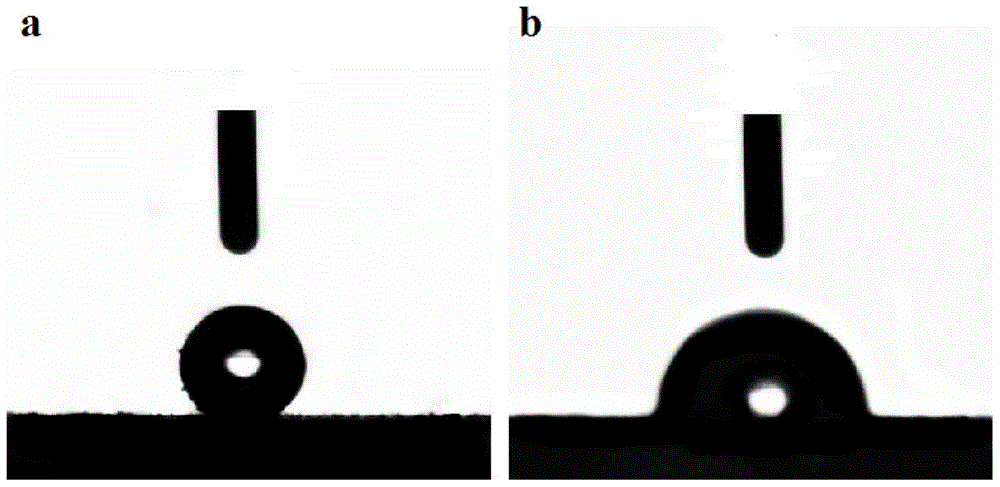
[0032] Depend on figure 1 a, b It can be seen that the organic solid catalyst before sulfonation is in the shape of a tube, the tube diameter is distributed in 80-100nm, and the two ends of the nanotube are open; figure 1 c It can be...
Embodiment 2
[0044] (1) Preparation of organic solid catalyst
[0045] Add 4g of styrene to 250g of hexane solvent, stir for 20-30min to make it evenly mixed, take 0.48g of BFEE into the mixed system, and react at 25°C for 120s, then add 10g of ethanol to terminate the reaction; Washing with methanol or ethanol and water for 3 to 5 times, drying under vacuum at 50 to 55°C for 20 to 24 hours to obtain a polymer with nanotubular hydrophobic properties;
[0046] Take 0.25g of the material obtained above and grind it into powder, add it to 50mL of 98% concentrated sulfuric acid solution, stir at 30°C for 6h, and vacuum dry at 70-80°C to obtain a nanotubular hydrophobic acidic catalyst.
[0047] (2) Analysis and test of catalytic performance
[0048]Catalytic performance analysis test method is the same as embodiment 1.
[0049] The results show that the yield of HMF is 38.2%, and the reaction time is 0.5h. The catalyst has higher catalytic performance, shorter catalytic time and less catalys...
Embodiment 3
[0054] (1) Preparation of Nanotubular Hydrophobic Acid Catalysts
[0055] Add 8g of vinylbenzyl chloride into 385g of dichloromethane solvent, stir for 20-30min to make it evenly mixed, take 0.96g of BFEE into the mixed system, and react at 40°C for 300s, then add 20g of ethanol to terminate the reaction; The product is washed with methanol or ethanol and water for 3 to 5 times, and dried under vacuum at 50 to 55°C for 20 to 24 hours to obtain a polymer with nanotubular hydrophobic properties;
[0056] Take 0.25g of the material obtained above, grind it into powder, add it to 50mL of 98% chlorosulfonic acid solution, stir at 50°C for 10h, and dry it in vacuum at 70-80°C to obtain a nanotubular hydrophobic acidic catalyst.
[0057] (2) Analysis and test of catalytic performance
[0058] Catalytic performance analysis test method is the same as embodiment 1.
[0059] The results show that the yield of HMF is 37%, and the reaction time is 0.5h. The catalyst has higher catalytic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com