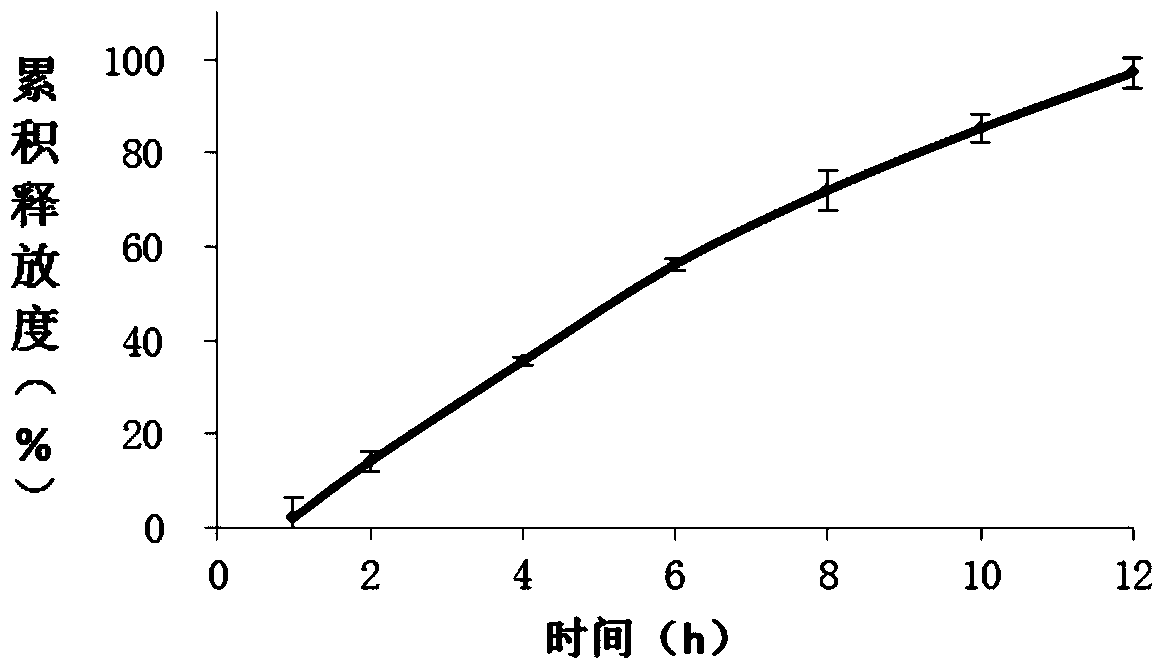
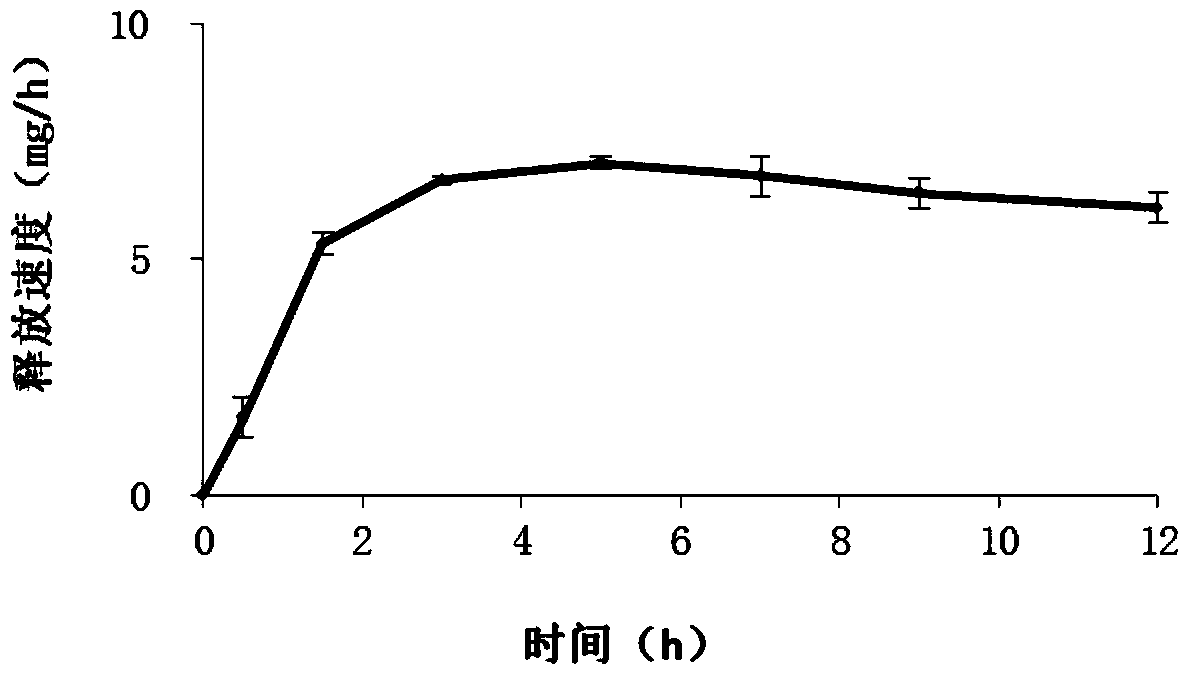
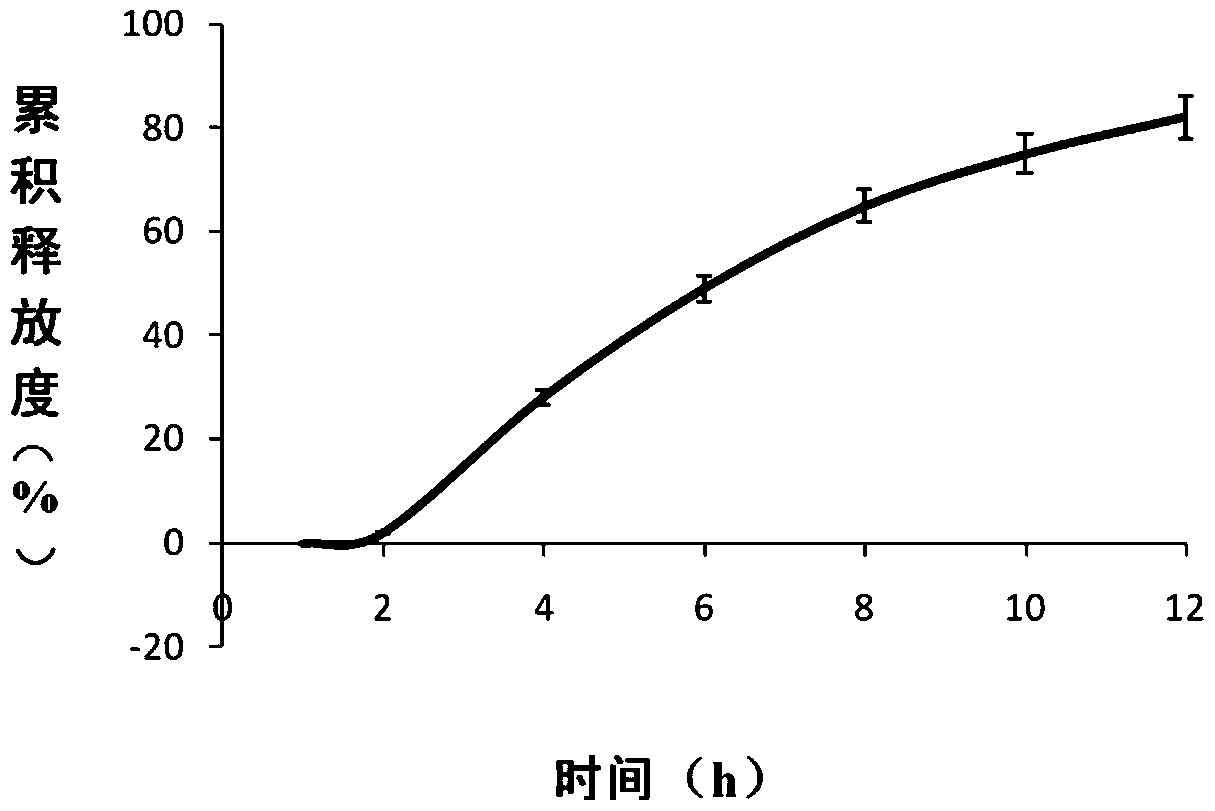
Ambroxol hydrochloride osmotic pump controlled-release tablet and preparation method thereof
A technology of ambroxol hydrochloride and osmotic pump controlled release, which is applied in the field of preparation of ambroxol hydrochloride osmotic pump controlled release tablets, and can solve the problem of decreased end release rate of ambroxol hydrochloride, unfavorable swallowing by patients, and incomplete drug release To achieve stable and long-lasting blood drug concentration, maintain blood drug concentration, and reduce the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation of embodiment 1 ambroxol hydrochloride osmotic pump controlled release tablet
[0058] (1) Drug-containing layer
[0059]
[0060] (2) booster layer
[0061]
[0062] (3) Coating solution
[0063] Cellulose acetate 40g
[0064] Polyethylene glycol 8g
[0065] Proper amount of acetone
[0066] (4) Prescription of moisture-proof coating solution
[0067] Gastric-soluble coating powder 18g
[0068] Purified water 82g
[0069] Made a total of 100g
[0070] Preparation of drug-containing layer granules: Weigh ambroxol hydrochloride, povidone, copovidone, sodium chloride, and iron oxide yellow according to the stated ratio, pulverize them through a 120-mesh sieve, mix well, and use a concentration of not less than 40% The adhesive soft material prepared by ethanol solution, 20-mesh sieve to make wet granules, dry at 50°C until the water content is 3-5%, granulate, add magnesium stearate and micro-powder silica gel in the proportion described abov...
Embodiment 2
[0075] The preparation of embodiment 2 ambroxol hydrochloride osmotic pump controlled-release tablets
[0076] (1) Drug-containing layer
[0077]
[0078]
[0079] (2) booster layer
[0080]
[0081] (3) Coating solution
[0082] Cellulose acetate 40g
[0083] Polyethylene glycol 6g
[0084] Proper amount of acetone
[0085] (4) Prescription of moisture-proof coating solution
[0086] Gastric-soluble coating powder 18g
[0087] Purified water 82g
[0088] Made a total of 100g
[0089] Preparation of drug-containing layer granules: Weigh ambroxol hydrochloride, povidone, poloxamer, sodium chloride, and iron oxide yellow according to the stated ratio, crush them through a 120-mesh sieve, mix well, and use a concentration of not less than 40 % ethanol solution to prepare soft materials made of adhesives, 20 mesh sieves to make wet granules, dry at 50°C until the water content is 3% to 5%, granulate, add magnesium stearate and micropowder silica gel in the stated ...
Embodiment 3
[0094] The preparation of embodiment 3 ambroxol hydrochloride osmotic pump controlled-release tablets
[0095] (1) Drug-containing layer
[0096]
[0097] (2) booster layer
[0098]
[0099] (3) Coating solution
[0100] Cellulose acetate 40g
[0101] Polyethylene glycol 8g
[0102] Proper amount of acetone
[0103] (4) Prescription of moisture-proof coating solution
[0104] Gastric-soluble coating powder 18g
[0105] Purified water 82g
[0106] Made a total of 100g
[0107] Preparation of drug-containing layer granules: Weigh ambroxol hydrochloride, povidone, polyoxyethylene, sodium chloride, and iron oxide yellow according to the stated ratio, crush them through a 120-mesh sieve, mix well, and use a concentration of not less than 40% The adhesive soft material prepared by ethanol solution, 20 mesh sieve to make wet granules, dry at 40°C until the water content is 3-5%, granulate, add magnesium stearate and micro-powder silica gel in the proportion described ab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com