Amphiphilic Eu (III) complex, preparation method thereof and application thereof in sensing identification of citric acid/isocitric acid
A complex and amphiphilic technology, used in fluorescence/phosphorescence, organic chemistry, material excitation analysis, etc., can solve the problems of different interactions, high experimental cost, narrow application range, etc., and achieve a large Stokes shift. , the preparation method is simple, the effect of Stokes shift coordination unsaturated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Synthetic formula I compound
[0052] 4.000g (16.2mmol) Boc-L-glutamic acid, 12.400g (64.8mmol) 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (WSC), 0.160g ( Add 1.3mmol) of 4-dimethylaminopyridine (DMAP) into 100mL of dichloromethane, stir to dissolve it completely, add 4.857g (40.5mmol) of n-hexylamine under ice bath conditions, continue stirring for 30 minutes, remove the ice bath, and The reaction was stirred for 24 hours, and the resulting clear solution was extracted with saturated NaCl aqueous solution, and the extracted organic phase was washed with anhydrous NaCl 2 SO 4 Drying, then rotary evaporation, column chromatography (the eluent is a mixture of chloroform and methanol in a volume ratio of 10:1), and vacuum drying to obtain a white solid, the compound of formula I, with a yield of 85.6%. The equation is as follows:
[0053]
[0054] The structural characterization data of the compound of formula I are: 1 H NMR (300MHz, CDCl 3 , Me ...
Embodiment 2
[0080] The use of amphiphilic Eu(Ⅲ) complexes in sensing and identifying citric acid and isocitric acid, the detection method is as follows:
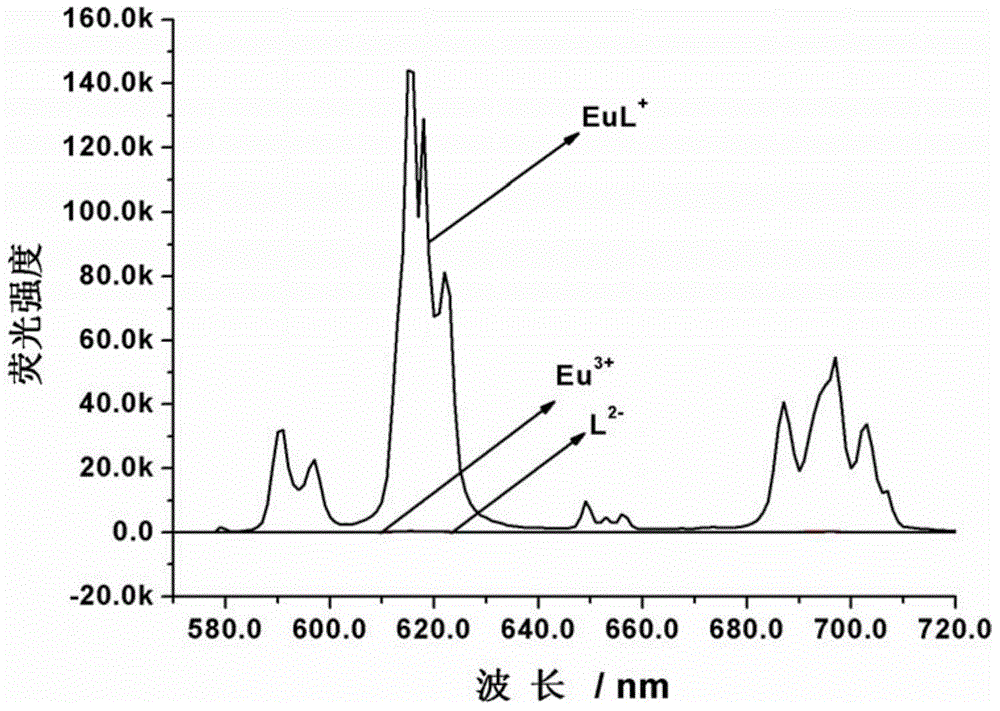
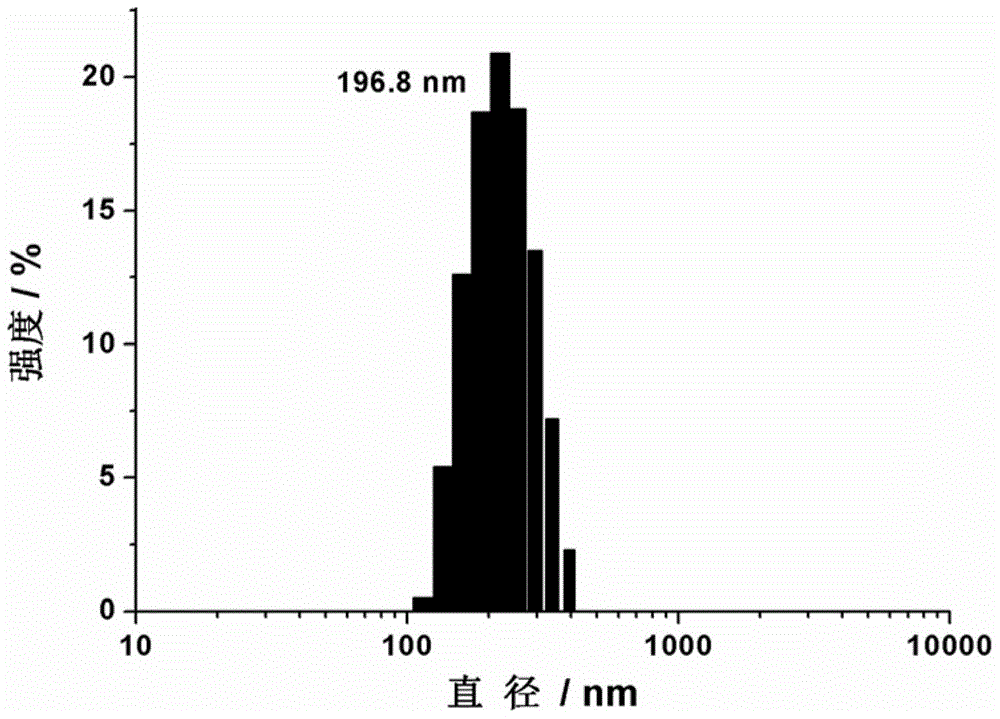
[0081] 1.68 mg (2 μmol) of the amphiphilic Eu(Ⅲ) complex was completely dissolved in 20 mL of 10 mmol / L 4-hydroxyethylpiperazineethanesulfonic acid buffer solution with a pH value of 7.4 to obtain 100 μmol / L amphiphilic Eu( III) The complex solution was kept at 60° C. for 2 hours, then naturally cooled to room temperature, and uniform vesicles were formed after 8 hours. The morphology of the formed vesicles was characterized by dynamic light scattering, scanning electron microscopy, and transmission electron microscopy, and the results are shown in Figure 2~4 . Depend on figure 2 It can be seen that the amphiphilic Eu(Ⅲ) complexes are spherical aggregates in the buffer solution with an average diameter of 196nm. At the same time by image 3 It is further confirmed that the aggregates are spherical structures. A more accurate micr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com