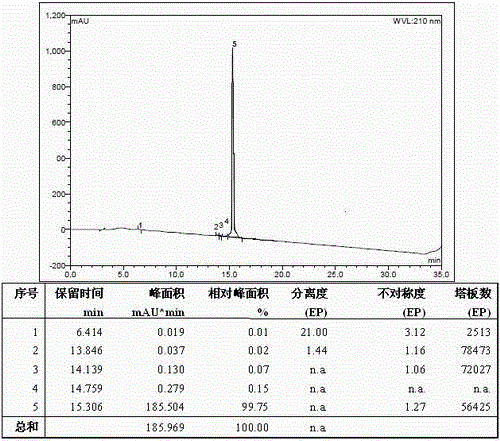
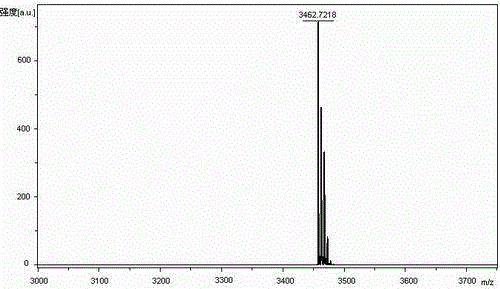
Method for preparing nesiritide
A technology of peptide fragment and liquid phase method, which is applied in the field of polypeptide drug preparation, and can solve problems such as low production efficiency, unsuitable for large-scale production, and incomplete coupling of arginine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: Fmoc-Arg(Boc) 2 Synthesis of -OSu Activated Ester
[0090] Weigh 596.67g Fmoc-Arg(Boc) 2 -OH (1.0mol), 138.10g HOSu (1.2mol) was added to 3000ml THF, 247.56g DCC (1.2mol) was added in an ice-water bath, reacted for 1 hour, warmed up to room temperature and reacted for 3 hours, the reaction solution was filtered, and the mother liquor was spin-dried, Add DCM to dissolve, filter, wash with saturated sodium bicarbonate 3 times, pure water 2 times, back-extract 2 times, combine organic phases, dry with anhydrous sodium carbonate, spin dry, recrystallize with ice ethanol 3 times, filter, and pull dry with solid oil pump Obtained 617.45g Fmoc-Arg(Boc) 2 -OSu activated ester, yield 89%.
Embodiment 2
[0091] Embodiment 2: Fmoc-Arg(Boc) 2 -Arg(Boc) 2 Synthesis of -OH
[0092] Weigh 187.33g H-Arg(Boc) 2 -OH (0.5mol) and 79.50g Na 2 CO 3 (0.75mol) was added to the mixed solution of 500ml water and 500ml THF to dissolve, and weighed 346.88g Fmoc-Arg(Boc) 2 -OSu (0.5mol) was added to 500ml THF, dissolved and added dropwise to the above mixed solution, reacted overnight at room temperature, adjusted the pH to 7 with 10% dilute hydrochloric acid, removed THF by rotary evaporation, and then adjusted the pH to 3. A large white precipitate was obtained which was filtered. The resulting white precipitate was recrystallized from ice ethanol. The solid oil pump was dried to obtain 414.70g Fmoc-Arg(Boc) 2 -Arg(Boc) 2 -OH, yield 87%.
Embodiment 3
[0093] Embodiment three: Fmoc-Arg (Boc) 2 -Arg(Boc) 2 Synthesis of -OSu Activated Ester
[0094] Weigh 381.34g Fmoc-Arg(Boc) 2 -Arg(Boc) 2-OH (0.4mol), 55.24g HOSu (0.48mol) was added to 800ml THF, 99.02g DCC (0.48mol) was added in an ice-water bath, reacted for 1 hour, warmed up to room temperature and reacted for 3 hours, the reaction liquid was filtered, and the mother liquor was spin-dried, Add DCM to dissolve, filter, wash with saturated sodium bicarbonate 3 times, pure water 2 times, back-extract 2 times, combine organic phases, dry with anhydrous sodium carbonate, spin dry, recrystallize with ice ethanol 3 times, filter, and pull dry with solid oil pump Obtained 373.95g Fmoc-Arg(Boc) 2 -Arg(Boc) 2 -OSu activated ester, yield 89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com