Phosphorylcholine-containing high-strength polyurethane hydrogel and preparation method thereof
A technology of phosphorylcholine and polyurethane, which is applied in the field of high-strength polyurethane hydrogel and its preparation, can solve the problems of not fully satisfying articular cartilage, unfavorable microphase separation structure, hindering the formation of multiple hydrogen bonds, etc., and achieve excellent biocompatibility The effect of high stability, excellent mechanical properties, and high-strength biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
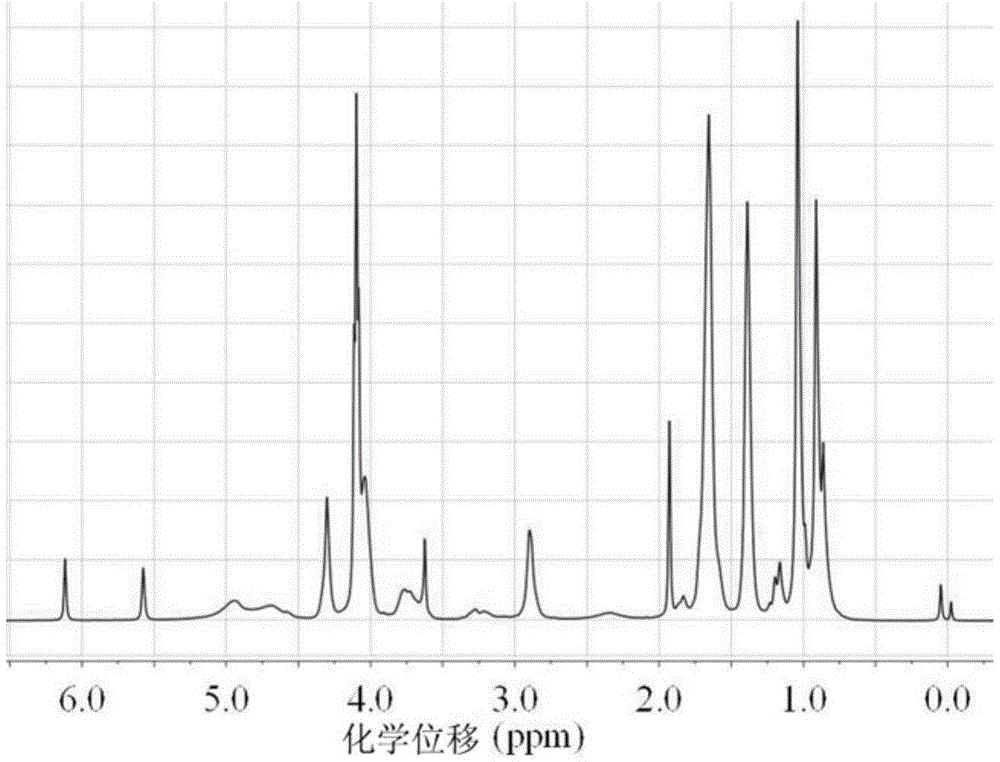
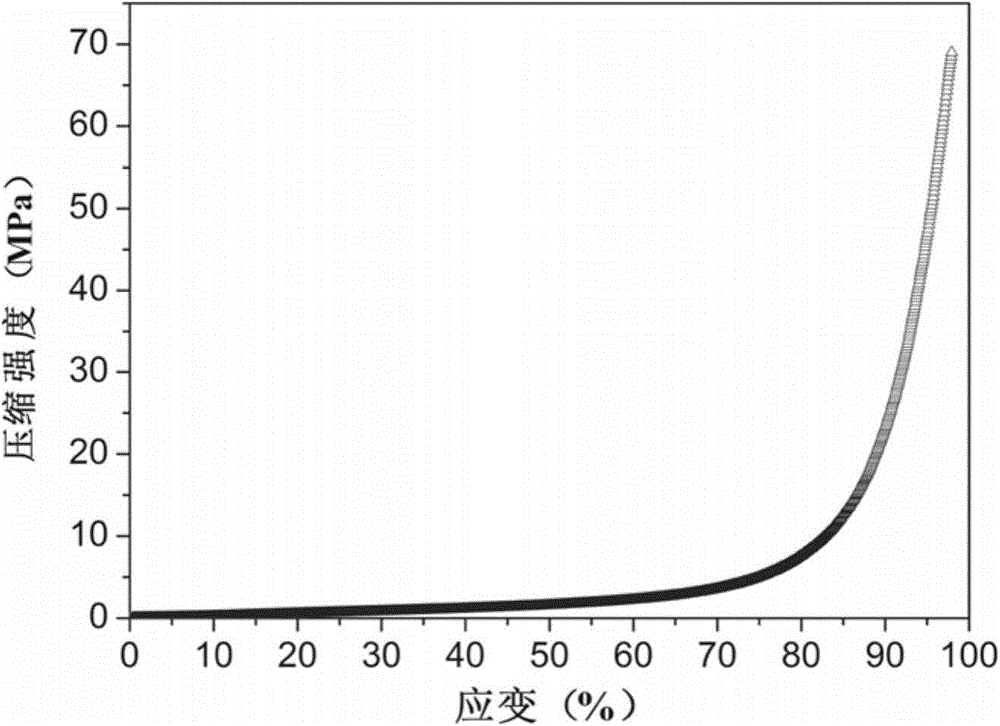
[0036] A high-strength polyurethane hydrogel containing phosphorylcholine. The polyurethane hydrogel is composed of a double bond-terminated polyurethane block copolymer, methacryloyloxyethyl phosphorylcholine, and olefinic comonomers through free radicals. Polymerized and copolymerized, the mass ratio of double bond-terminated polyurethane block copolymer to methacryloyloxyethyl phosphorylcholine is 12; The total addition of choline and olefinic comonomer accounts for 15% of the total mass of the reaction system.
[0037] The double-bond-terminated polyurethane block copolymer is prepared by stepwise addition polymerization including diisocyanate compounds, polyester diol, 1,4-butanediol, and hydroxyethyl methacrylate. The ethylenic comonomer was polyethylene glycol methyl methacrylate.
[0038] When preparing high-strength polyurethane hydrogel containing phosphorylcholine, the double-bond-terminated polyurethane block copolymer was prepared first, and then mixed with metha...
Embodiment 2
[0052] A high-strength polyurethane hydrogel containing phosphorylcholine. The polyurethane hydrogel is composed of a double bond-terminated polyurethane block copolymer, methacryloyloxyethyl phosphorylcholine, and olefinic comonomers through free radicals. Polymerization and copolymerization. The mass ratio of double-bond-terminated polyurethane block copolymer to methacryloyloxyethyl phosphorylcholine is 4; The total amount of comonomer added accounts for 15% of the total mass of the reaction system. The ethylenic comonomer was polyethylene glycol methyl methacrylate.
[0053] The preparation of the hydrogel specifically includes the following steps:
[0054] (1) Preparation of double-bond-terminated polyurethane block copolymer: same as in Example 1.
[0055] (2) Preparation of precursor solution:
[0056] At room temperature, 0.208 g of polyurethane block copolymer terminated with double bonds, 0.052 g of methacryloyloxyethyl phosphorylcholine, 0.042 g of polyethylene ...
Embodiment 3
[0060] A high-strength polyurethane hydrogel containing phosphorylcholine. The polyurethane hydrogel is composed of a double bond-terminated polyurethane block copolymer, methacryloyloxyethyl phosphorylcholine, and olefinic comonomers through free radicals. Polymerization and copolymerization. The mass ratio of double-bond-terminated polyurethane block copolymer to methacryloyloxyethyl phosphorylcholine is 12; The total amount of comonomer added accounts for 10% of the total mass of the reaction system. The ethylenic comonomer is N-isopropylacrylamide.
[0061] The preparation of the hydrogel specifically includes the following steps:
[0062] (1) prepare the polyurethane block copolymer of double bond termination:
[0063] Add diisocyanate compounds, polyester diol, 1,4-butanediol and dioctyltin dilaurate into a dry 250mL four-neck flask, stir and react at 82°C for 4.5 hours under the protection of argon, add N,N- Dimethylacetamide, lower the temperature to 45°C, slowly a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Compressive strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com