Preparation method of polycarbonate
A polycarbonate, copolymerization reaction technology, applied in the polymer field, can solve the problem of low catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a kind of preparation method of polycarbonate, comprises the following steps:
[0028] Under the action of the main catalyst and the co-catalyst, the carbon dioxide and the epoxide are copolymerized to obtain polycarbonate;
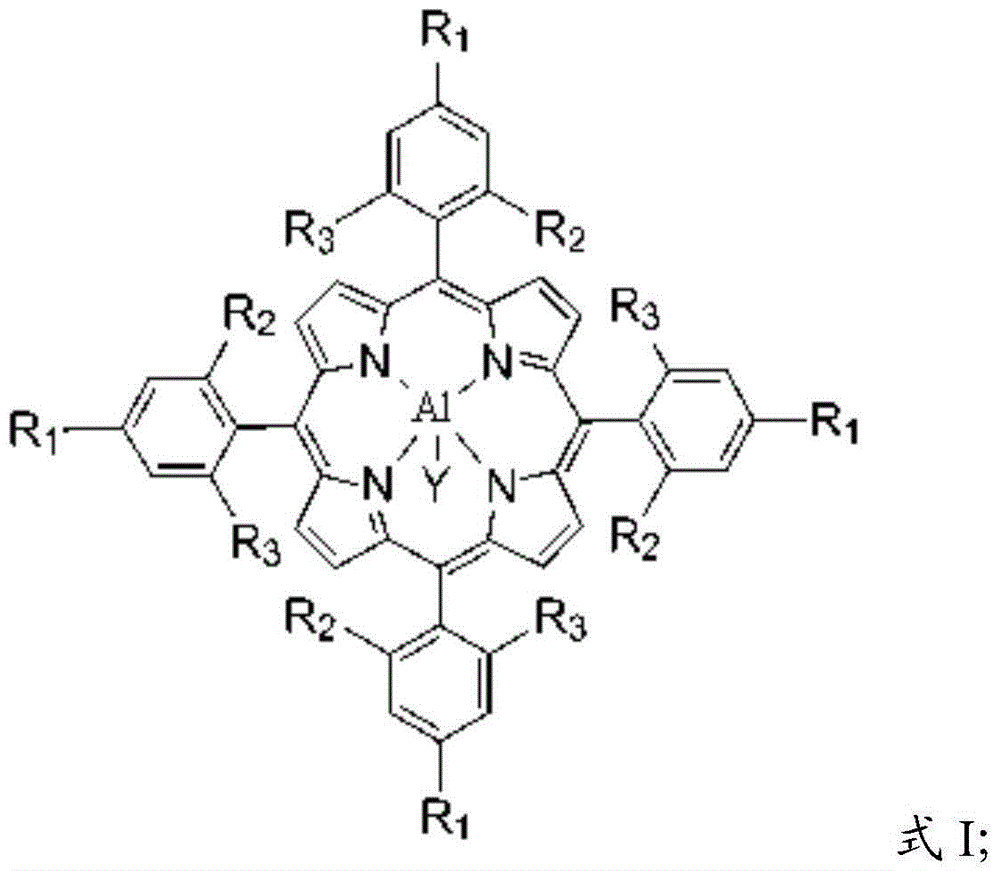
[0029] The main catalyst is an aluminum porphyrin complex, and the aluminum porphyrin complex has a structure shown in formula I:
[0030] Formula I;
[0031] In formula I, the R 1 selected from halogen, aliphatic, substituted aliphatic, substituted heteroaliphatic, aryl, substituted aryl, or substituted heteroaryl;
[0032] The R 2 and R 3 independently selected from hydrogen, halogen, aliphatic, substituted aliphatic, substituted heteroaliphatic, aryl, substituted aryl, or substituted heteroaryl;
[0033] The Y is a halogen group, -NO 3 、CH 3 COO-, CCl 3 COO-, CF 3 COO-, ClO 4 -, BF 4 -, BPh 4 -, -CN, -N 3 , p-toluate, p-toluenesulfonate, o-nitrophenol oxyanion, p-nitrophenol oxyanion, m-nitrophenol oxyanion,...
Embodiment 1
[0110] at 25°C, N 2 Under protection, add 800mL dry dichloromethane, 2.8g 4-chlorobenzaldehyde (20mmol), 1.4mL pyrrole (20mmol) into a 1000mL round-bottomed three-neck flask, stir until the solid is completely dissolved, then add 3.7mL trifluoroacetic acid ( 50mmol, 2.5eq) and stirred for 1h, then added 9.08g DDQ (2.3-dichloro-5,6-dicyano-1,4-benzoquinone) and stirred for 1h. The above liquid was filtered under reduced pressure, the solvent was distilled off under reduced pressure, and the obtained crude product was separated by chromatographic column [stationary phase: aluminum oxide; mobile phase: dichloromethane / petroleum ether (volume ratio)=2:1] to obtain four (4-chlorophenyl) porphyrin, product conversion rate 20.8%; The present invention carries out proton nuclear magnetic resonance spectrum test to the tetrakis (4-chlorophenyl) porphyrin obtained, test result shows: 1 H NMR (300MHz, CDCl3), δ: 8.83(s, 8H), 8.12(d, J=9.0Hz, 8H), 7.78(d, J=9.0Hz, 8H), -2.87(s, 2H); iden...
Embodiment 2
[0114] at 25°C, N 2 Under protection, add 800mL of dry dichloromethane, 3.7g of 4-bromobenzaldehyde (20mmol), and 1.4mL of pyrrole (20mmol) into a 1000mL round-bottomed three-neck flask, stir until the solid is completely dissolved, then add 3.7mL of trifluoroacetic acid ( 50mmol, 2.5eq) and stirred for 1h, then added 9.08g DDQ (2.3-dichloro-5,6-dicyano-1,4-benzoquinone) and stirred for 1h. The above liquid was filtered under reduced pressure, the solvent was distilled off under reduced pressure, and the obtained crude product was separated by chromatographic column [stationary phase: aluminum oxide; mobile phase: dichloromethane / petroleum ether (volume ratio)=2:1] to obtain four (4-bromophenyl) porphyrin, product conversion rate 19.5%; The present invention carries out proton nuclear magnetic resonance spectrum test to the tetrakis (4-bromophenyl) porphyrin obtained, and test result is: 1 H NMR (300MHz, CDCl3), δ: 8.84 (s, 8H), 8.06 (d, J = 9.0Hz, 8H), 7.91 (d, J = 9.0Hz, 8H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com