Carbon dioxide based high polymer material with crystallization performance
A technology of polymer materials and carbon dioxide, applied in the field of carbon dioxide-based polymer materials, can solve problems such as being limited within its glass transition temperature, and polycarbonate materials having no crystalline properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] In a pre-dried stainless steel autoclave with an effective volume of 200mL and at ambient temperature, add in the following order: 0.5×10 -3 Mole of catalyst 1 in S configuration, X is 2,4-dinitrophenol anion), 0.5×10 -3 Mole of two-(triphenylphosphine, 2,4-dinitrophenol ammonium oxide, 0.25 mole of refined R-configuration styrene oxide, the temperature is controlled at -10 degrees, and then carbon dioxide gas is introduced and kept 2.0MPa constant pressure, react under magnetic stirring for 24 hours, slowly release unreacted carbon dioxide in the autoclave, add a large amount of ether to precipitate polycarbonate. Filter, wash with ether several times, vacuum dry to constant weight, and obtain 20 grams Polystyrene carbonate white solid.The average molecular weight of polymer is 18000 by gel permeation chromatography, and molecular weight distribution is 1.12; Measure its with Varian INOVA-400MHz nuclear magnetic resonance instrument 1 H-NMR, found that the...
Embodiment 2
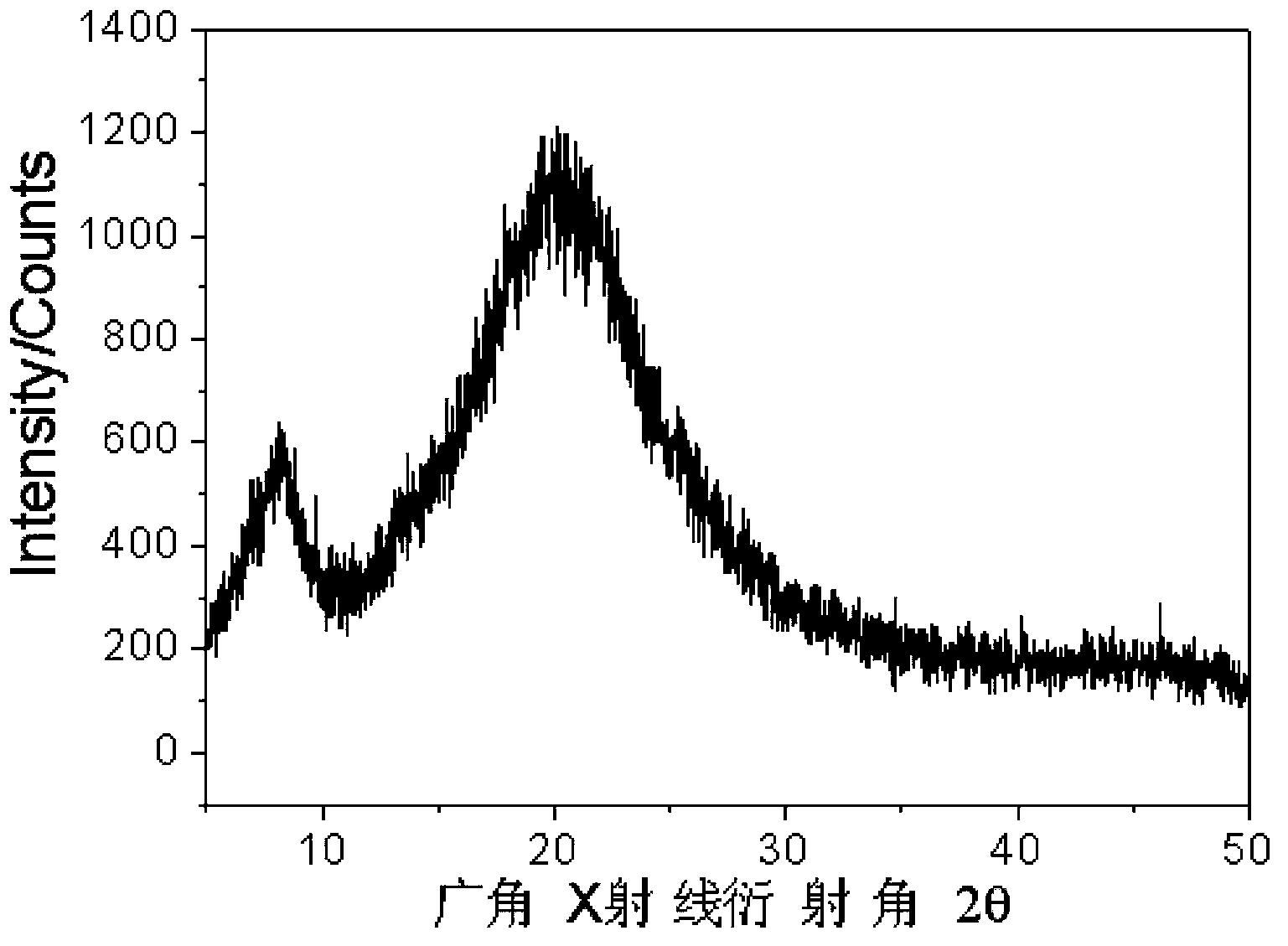
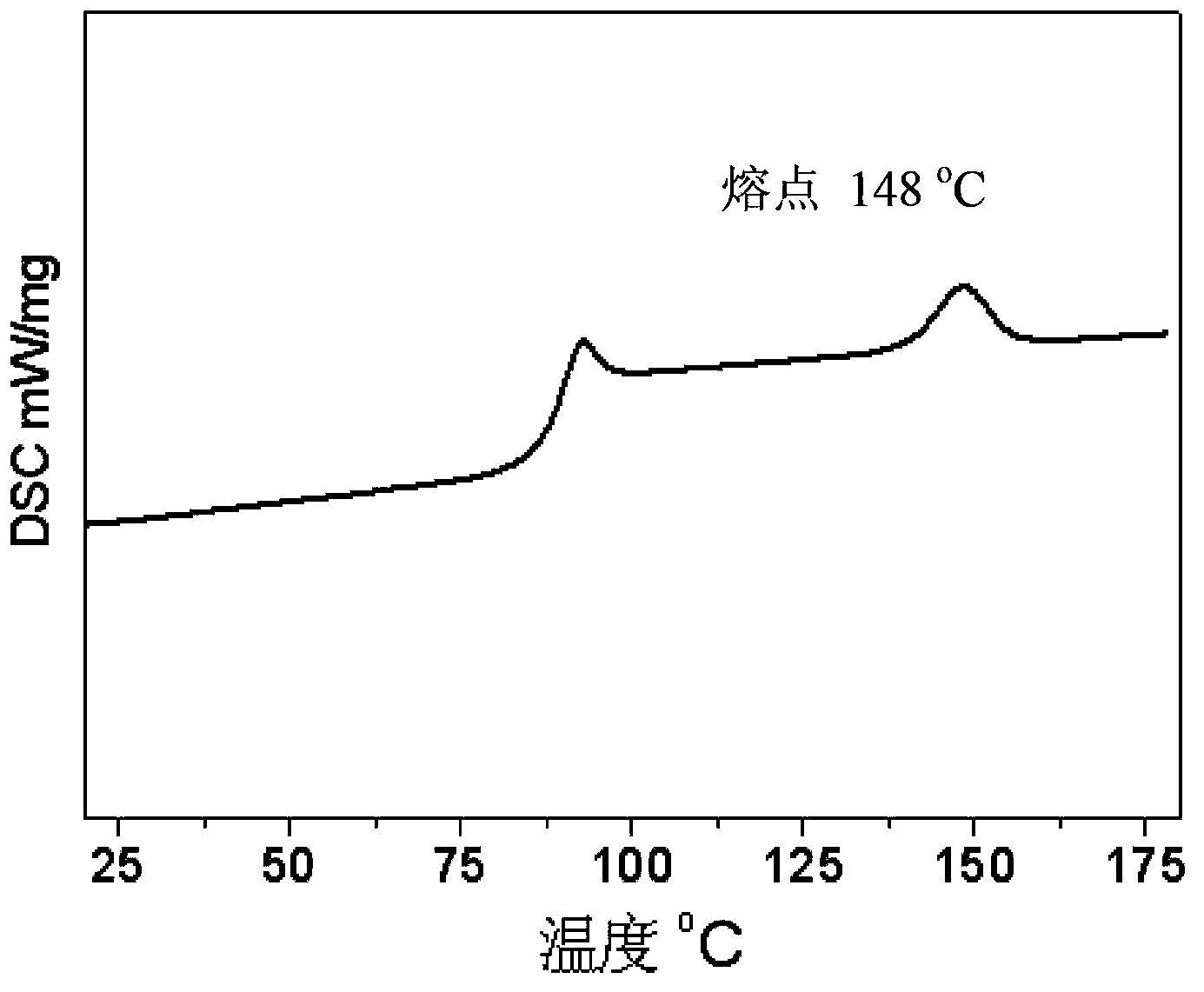
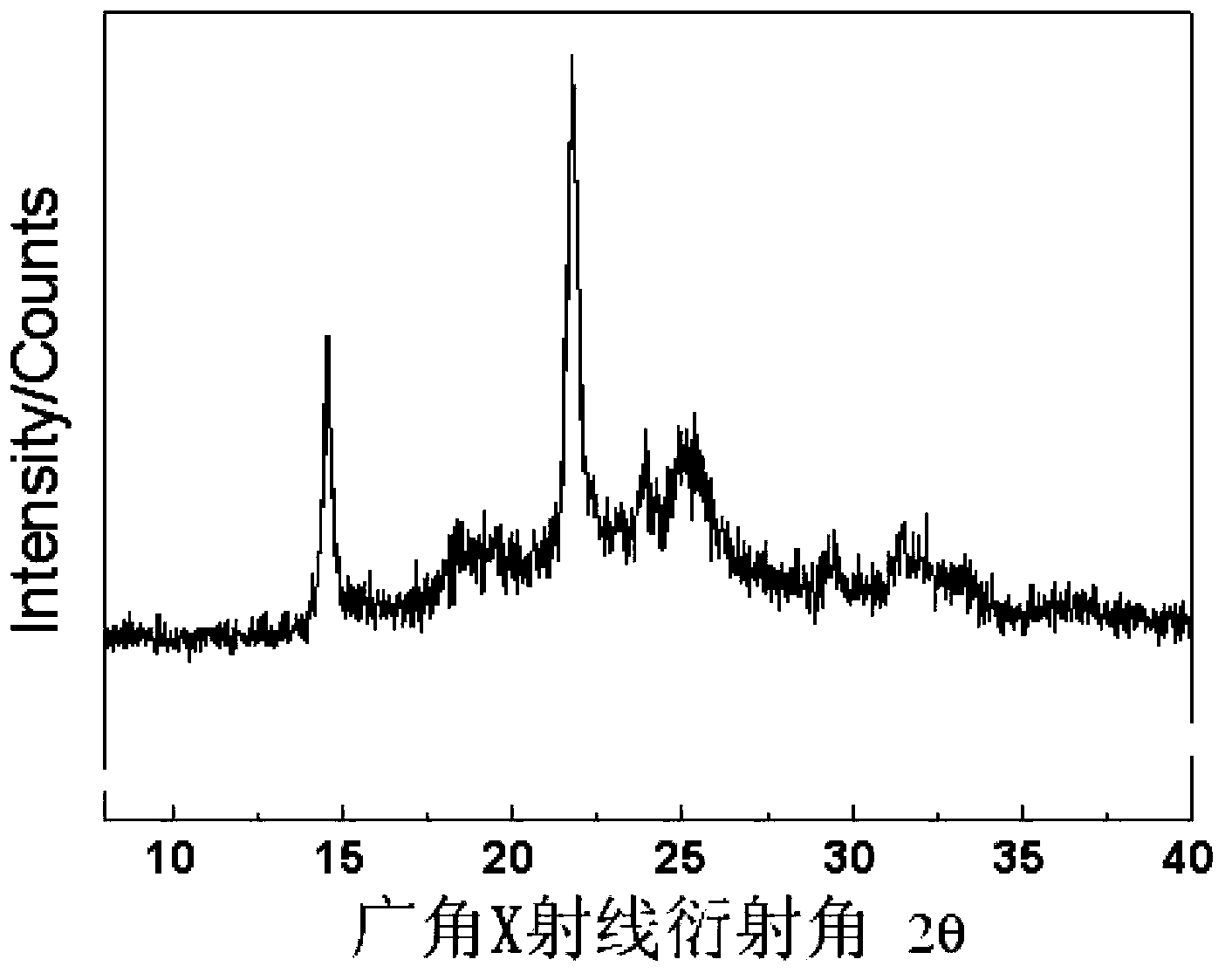
[0049] In the same equipment used in Example 1, under the same conditions, only the temperature was lowered to -20 ° C for 48 hours to obtain 14 grams of polystyrene carbonate with a molecular weight of 12000 and a molecular weight distribution of 1.15. Polymerization The content of carbonate units in the product reaches 99%. pass 13 C-NMR analysis found that the isotactic structure of the polymer was more than 95%, and a small amount of polycarbonate hydrolyzed was analyzed by chiral gas phase column, and the enantioselectivity of the obtained 1-phenyl 1,2-ethylene glycol was 96% (S / R=98 / 2); After the obtained polymer was kept at a constant temperature of 130°C for 5 minutes, it was analyzed by DSC, and it was found that the glass transition temperature of the polymer was 93°C, and the maximum crystalline melting peak appeared at 148°C. The enthalpy is 27.1J / g; thermogravimetric analysis shows that the decomposition temperature of 50% of the material is 310°C; the wide-angl...
Embodiment 3
[0051]
[0052] In a pre-dried stainless steel autoclave with an effective volume of 200mL and at ambient temperature, add in the following order: 0.5×10 -3 moles of S-configuration catalyst 2, X and Y are 2,4-dinitrophenolate anion), 0.25 moles of refined R-configuration epichlorohydrin, the temperature is controlled at 0 degrees, and then carbon dioxide is introduced Gas and maintain a constant pressure of 2.0MPa, react under magnetic stirring for 24 hours, slowly release unreacted carbon dioxide in the autoclave, add a large amount of ether to precipitate polycarbonate. Filter, wash with ether several times, and dry in vacuum to constant weight to obtain 20 g of polycarbonate as a white solid. The average molecular weight of polymer measured by gel permeation chromatography is 18000, and molecular weight distribution is 1.12; Measure its with Varian INOVA-400MHz nuclear magnetic resonance instrument 1 H-NMR, found that the alternate structure exceeds 99%, by 13 C-NMR a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enthalpy of fusion | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com