Preparation method of phage display-expressing circovirus antigen vaccine
A phage display, circovirus technology, applied in the direction of viral antigen components, antiviral immunoglobulins, viral peptides, etc., can solve the problems of complex methods, little effect and high cost, and achieve easy preparation, good protection effect, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Acquisition of porcine circovirus type Ⅱ ORF2 gene and preparation of polyclonal antibody for immune screening
[0022] 1 Acquisition of porcine circovirus type Ⅱ ORF2 gene.
[0023] Search the ORF2 sequence of PCV2 on GENBANK, search for the open reading frame according to the obtained sequence, and design the following primers according to the sequence of the open reading frame:
[0024] Upstream primer 5'-CGGGTACCGCCACCATGACGTATCCAAGGAGGC-3'
[0025] Downstream primer 5'-GCGGATCCTCACTTAGGGTTAAGTGGGGGG-3'
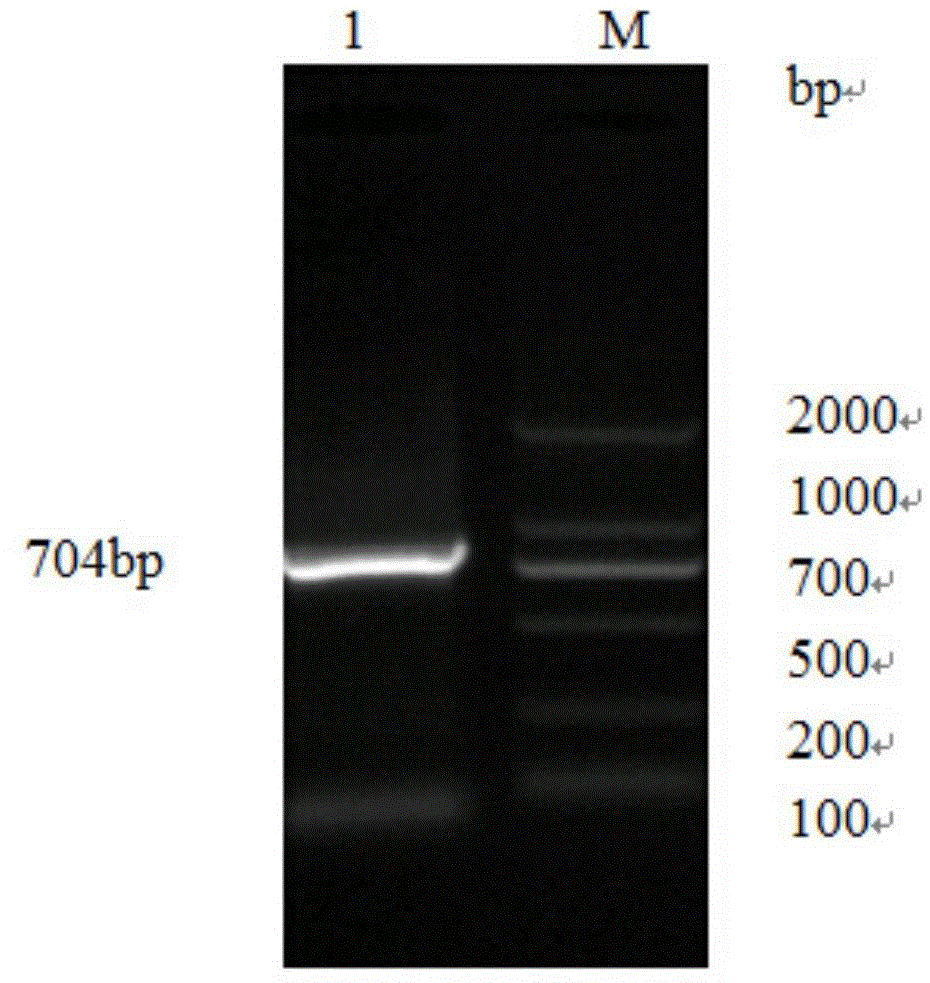
[0026] Extract the mRNA of porcine circovirus type Ⅱ virus culture solution, reverse transcribe it into cDNA, and use this as a template to amplify the ORF2 fragment by PCR. The amplification results of the target gene are as follows: figure 1 As shown, 704bp is the target band, connected to the PMD-18T vector, and the characteristics of the gene fragment obtained by sequencing are as follows:
[0027] The length of the ORF is 704bp, the start codon is...
Embodiment 2
[0037] Example 2 Insertion of porcine circovirus type Ⅱ ORF2 gene into T4 phage
[0038] After the porcine circovirus type II ORF2 gene fragment was ligated and inserted into the pMD-18T vector, it was digested with EcoR Ⅰ and hindIII to recover the digested fragment, and the T4 phage expression plasmid was digested with EcoRI and hindIII. The digested fragment was recovered, T4 ligase was used to connect the gene fragment to the SOC site of T4 phage, and the ligated recombinant T4 phage was used to infect X-Blue ST1 CCTCC M 2014397 host cells.
Embodiment 3
[0039] Example 3 Immune Screening
[0040] 1 Preparation of reagents
[0041] LB Tetracycline: add 10mg tetracycline to every 1mL of distilled water, dissolve and sterilize with a 0.22μm filter, store at -20°C.
[0042] Maltose-supplemented LB medium: 1L of LB medium, 0.2Mpa at 121°C, 20min, add 1M MgSO4 (10mL) and 2M maltose (3mL) filtered through a 0.22μm filter.
[0043] NZY agar: NaCl 5g, MgSO 4 .7H 2 O 2g, yeast extract 5g, hydrolyzed casein (NZ Qmine) 10g, agar 15g. Add deionized water to 1L, adjust pH=7.5, 121℃0.2Mpa, 20min, after cooling down to room temperature, pour into a petri dish.
[0044] NZY medium: NaCl 5g, MgSO 4 .7H 2 O 2g, yeast extract 5g, NZ Qmine 10g. Add deionized water to 1L, adjust pH=7.5, 121℃0.2Mpa, 20min.
[0045] NYZ top agar: NaCl 5.8g, MgSO 4 .7H 2 O 2g, 1M Tris-HCl (pH=7.5) 50.0ml, 2% (w / v) gelatin 0.5ml. Prepare 1L of NYZ sterilized medium, add 0.7% (w / v) agarose, 0.2Mpa, 0.2Mpa at 121°C, and sterilize for 20min.
[0046] SM buffer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com