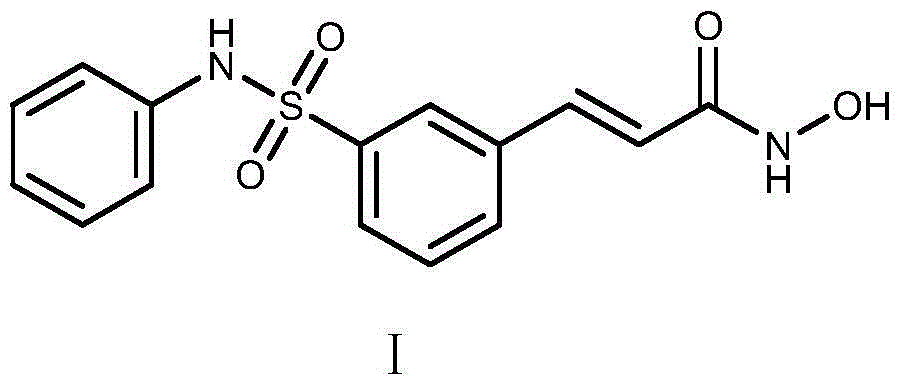
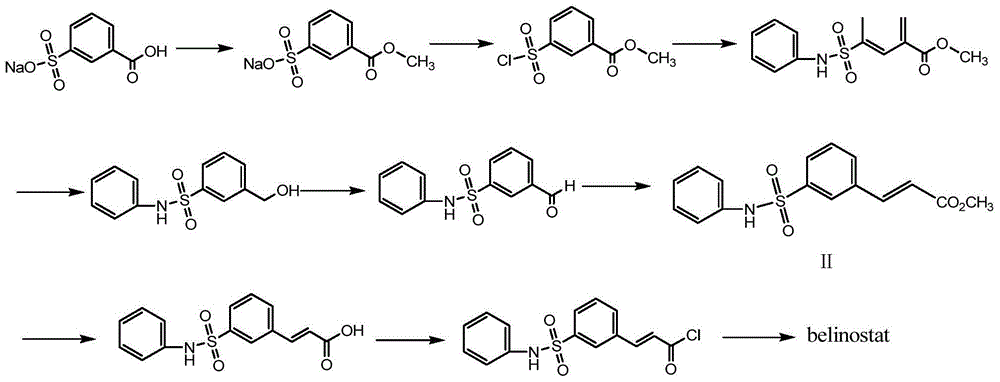
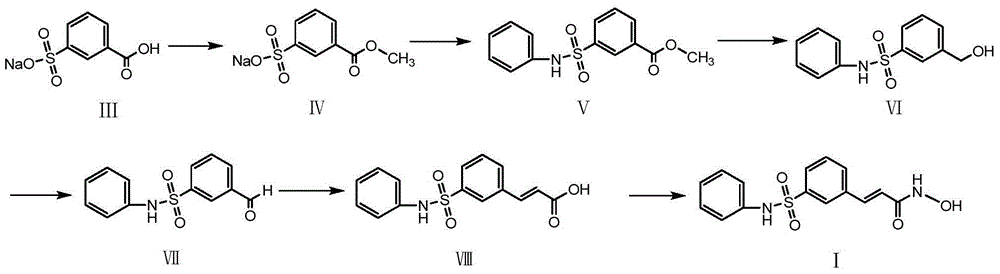
Belinostatsynthesis method suitable for industrial production
A synthetic method, the technology of belistat, applied in the direction of organic chemistry, sulfonamide preparation, etc., can solve the problems of easy decomposition and deterioration, long production cycle, easy to catch fire, etc., and achieve the goal of shortening the process cycle, simplifying the operation, and shortening the time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 compound IV
[0031]Add compound III, sodium m-carboxybenzenesulfonate (224g), methanol (3000ml) and hydrochloric acid (20g) into a 5L reaction flask, heat to reflux for 3h, cool down to room temperature, and concentrate to dryness to obtain compound IV, which is directly carried out to the next step.
Embodiment 2
[0032] The preparation of embodiment 2 compound V
[0033] Put the crude compound IV (2kg) into the reaction kettle, then add pyridine (6L), stir to dissolve, add thionyl chloride (1.2kg) dropwise, control the temperature at 40-50°C for 2-3h, add acetonitrile (6L) Dilute, lower the temperature to 0°C, add aniline (940g) dropwise, control the temperature below 30°C and react for 2 hours. After the reaction is completed, add it to a 50L reactor with 30L ice water, stir rapidly for 1h, filter, wash the filter cake with water, and then Wash with an appropriate amount of methyl tert-butyl ether / petroleum ether (1 / 2), suck dry, and dry at 50°C for 8 hours to obtain 1.75 kg of compound V with a yield of 71.5%.
Embodiment 3
[0034] The preparation of embodiment 3 compound VI
[0035] Add lithium chloride (848g) and potassium borohydride (1080g) into the reaction flask, add THF (12.5L) and stir to dissolve, heat at 50°C and stir for 30min, add the THF solution of compound V (3kg) dropwise, after the reaction is complete, add Add 4 L of ethyl acetate to 5.5 L of ice water, stir for 30 min, separate the liquid, separate the organic phase, dry, and concentrate to obtain the crude compound V; then recrystallize with toluene, and dry the solid at 50°C for 6 hours to obtain 2453 g of white crystalline compound VI , yield 90.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com