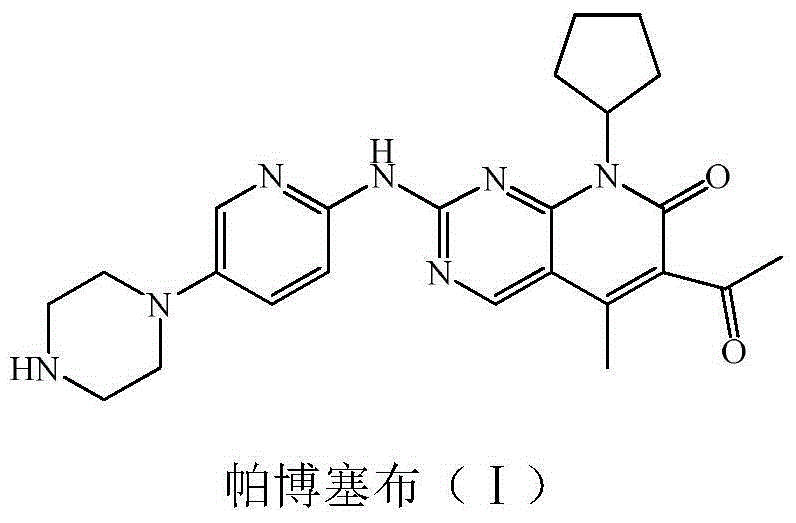
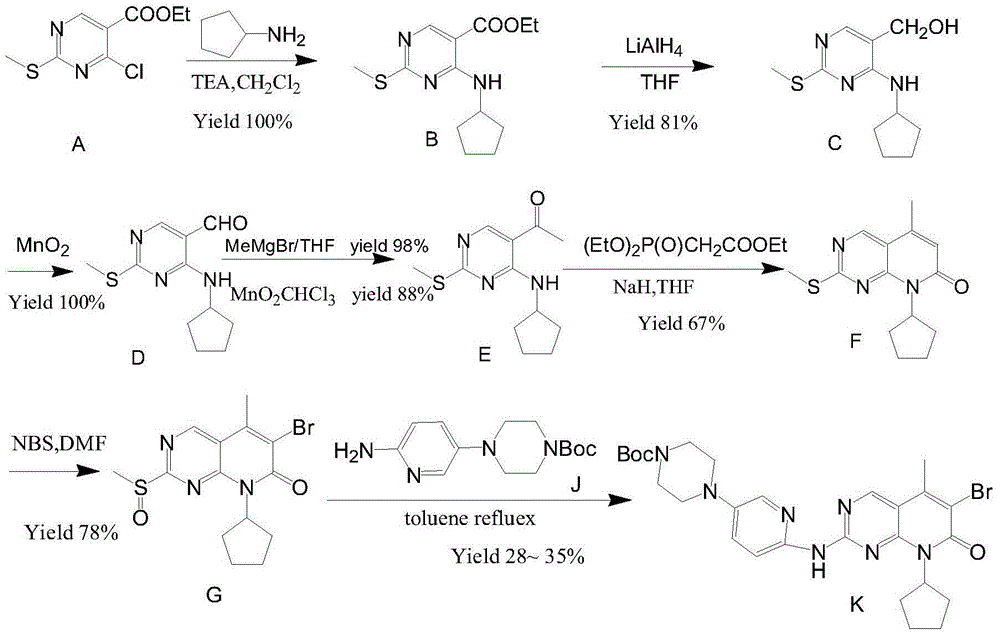
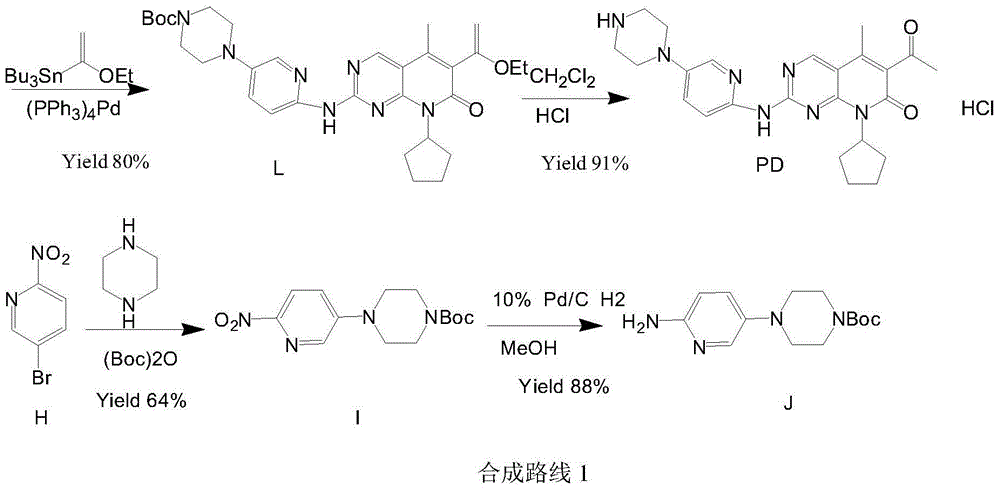
Preparation method of palbociclib
The technology of Paboseb and solvent is applied in the field of preparation of Paboseb, which can solve the problems of difficult industrialized operation, increase synthesis cost and the like, and achieve the effects of being beneficial to industrialized production, easy to operate, safe and environmentally friendly in process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of N,N-bis(acetoacetyl)cyclopentylamine (II)
[0058] 250 grams of tetrahydrofuran, 42.6 grams (0.5 moles) of cyclopentylamine, 2.5 grams of ammonium chloride, and 143.2 grams (1.1 moles) of ethyl acetoacetate were successively added to a 500 milliliter flask, and the reaction was maintained at 20 to 25 ° C for 6 hours. Recover tetrahydrofuran under reduced pressure below 40°C, cool to 20°C, add 200 grams of water, 300 grams of toluene, and 10 grams of sodium carbonate, stir for 30 minutes and separate layers, extract the water layer with toluene twice, each time with 50 grams of toluene, and combine the toluene layer, recovered toluene by distillation, and obtained 123.5 g of light yellow viscous liquid N,N-di(acetoacetyl)cyclopentylamine (II), with a yield of 97.6% and a gas phase purity of 98.6%.
Embodiment 2
[0059] Example 2: Preparation of N,N-bis(acetoacetyl)cyclopentylamine (II)
[0060] Replace 143.2 grams (1.1 moles) ethyl acetoacetate of embodiment 1 with 127.5 grams (1.1 moles) of methyl acetoacetate, replace the 2.5 grams of ammonium chloride of embodiment 1 with 3.0 grams of ammonium sulfate, all the other are with embodiment 1, obtain 124.6 g of light yellow viscous liquid N,N-di(acetoacetyl)cyclopentylamine (II), yield 98.4%, gas phase purity 98.5%.
Embodiment 3
[0061] Example 3: Preparation of N,N-bis(acetoacetyl)cyclopentylamine (II)
[0062] Add 250 g of 1,2-dichloroethane, 42.6 g (0.5 mole) of cyclopentylamine, 2.5 g of ammonium chloride, and 92.5 g of diketene into a 500 ml flask in sequence, and keep the reaction at 15 to 20°C for 6 hours. Recover tetrahydrofuran under reduced pressure below 40°C, cool to 20°C, add 200 g of water, 300 g of toluene, and 10 g of sodium carbonate, stir for 30 minutes and then separate into layers, extract the water layer twice with toluene (total 100 g of toluene), and combine the toluene layer, and recovered toluene by distillation to obtain 118.6 g of light yellow viscous liquid N,N-bis(acetoacetyl)cyclopentylamine (II), with a yield of 93.7% and a gas phase purity of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com