Preparation method for recombinant ubiquitin blue ear disease vaccine
A blue-ear disease and ubiquitination technology, applied in the field of biotechnology genetic engineering, can solve problems such as the inability to effectively remove viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
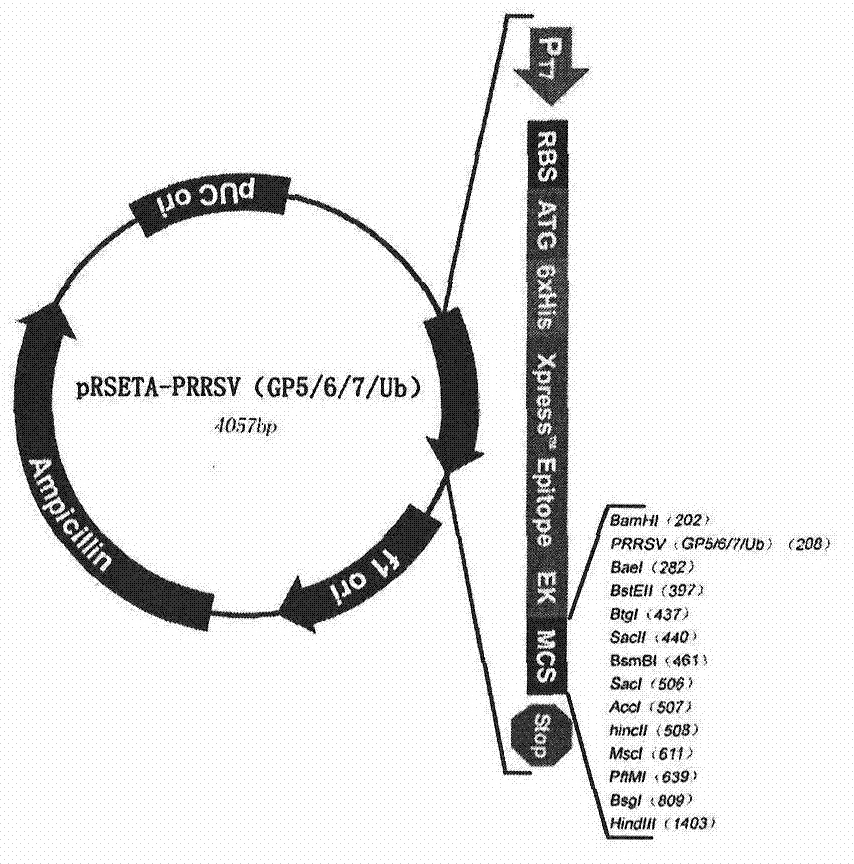
[0024] Example Escherichia coli expression vector and the construction of expression strain
[0025]The designed polypeptide-encoding nucleotides were sent to Shanghai Handsome Biotechnology Co., Ltd. for synthesis. BamH I (5' end) and HindIII (3' end) restriction enzyme sites were designed at both ends of the nucleotide fragment. After synthesis, they were respectively cloned into the pMD18T vector, and sequence determination confirmed that the inserted gene fragment was consistent with the designed sequence (see the sequence list). The recombinant plasmids were named pMD18T-PRRSV (GP5 / 6 / 7 / Ub). The two plasmids were digested with the corresponding restriction enzymes. The Escherichia coli expression vector was the pRSETA plasmid from Invitrogen Company, and the same restriction enzymes were also used for treatment. Digestion conditions: 10 μl reaction system, adding 2 μl of plasmid, 5 activity units of restriction endonuclease (New England biolabs), 1 μl of 10× buffer was ad...
Embodiment 3
[0029] Fermentation, purification and emulsification of embodiment three engineering bacteria
[0030] The production strains were taken for fermentation, inoculated into 2ml LB liquid medium (containing 100 μg / ml ampicillin), and cultured at 37° C. with shaking at 180 rpm for 12 hours to activate the strains. Then inoculate the shake flask with an inoculation amount of 1:100, shake and culture at 37°C until OD600=3, and then inoculate it into a fermenter at a ratio of 10%. The medium used for fermentation is a semi-synthetic medium prepared with distilled water and does not contain any antibiotics. Calibrate the dissolved oxygen and pH electrodes, start the tank to stir, the rotation speed is 300rpm, and sterilize the tank on-line. When the temperature of the culture solution in the tank drops to 37.0°C, calibrate the pH and dissolved oxygen (OD) zero point. The fermentation temperature is 37.0±0.1°C, the dissolved oxygen is controlled at about 40%, and the pH is controlled ...
Embodiment 4
[0033] Example 4 Safety Test of Recombinant Ubiquitinated PRRS Vaccine
[0034] Safety of Vaccines in Mice
[0035] Inject 18-22g Balb / C mice subcutaneously, 0.5ml each, and inject 5 mice in each batch of vaccine, a total of three batches, inject 15 mice, and set 2 negative controls at the same time, observe continuously for 10 days, observe Health status of mice.
[0036] Safety of Vaccines in Piglets
[0037] Select 30-day-old healthy three-element hybrid piglets, and inject recombinant ubiquitinated PRRS vaccine intramuscularly behind each ear, 5 piglets in each batch, a total of three batches, 15 piglets were injected, and 2 negative controls were set up at the same time. Head injection of physiological saline white oil emulsion 2ml, clinical observation for 10 days.
[0038] result
[0039] Safety Test of Vaccine on Mice
[0040] The results are shown in Table 1. The body temperature of one animal in the 20120208 immunization group rose slightly on the second day, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com