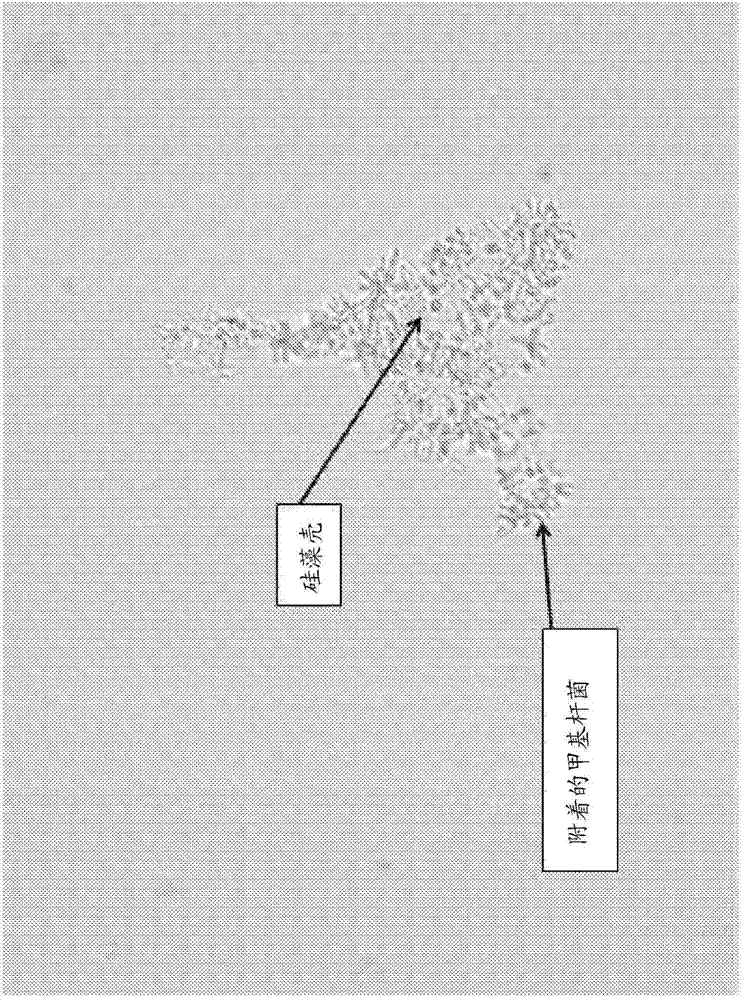
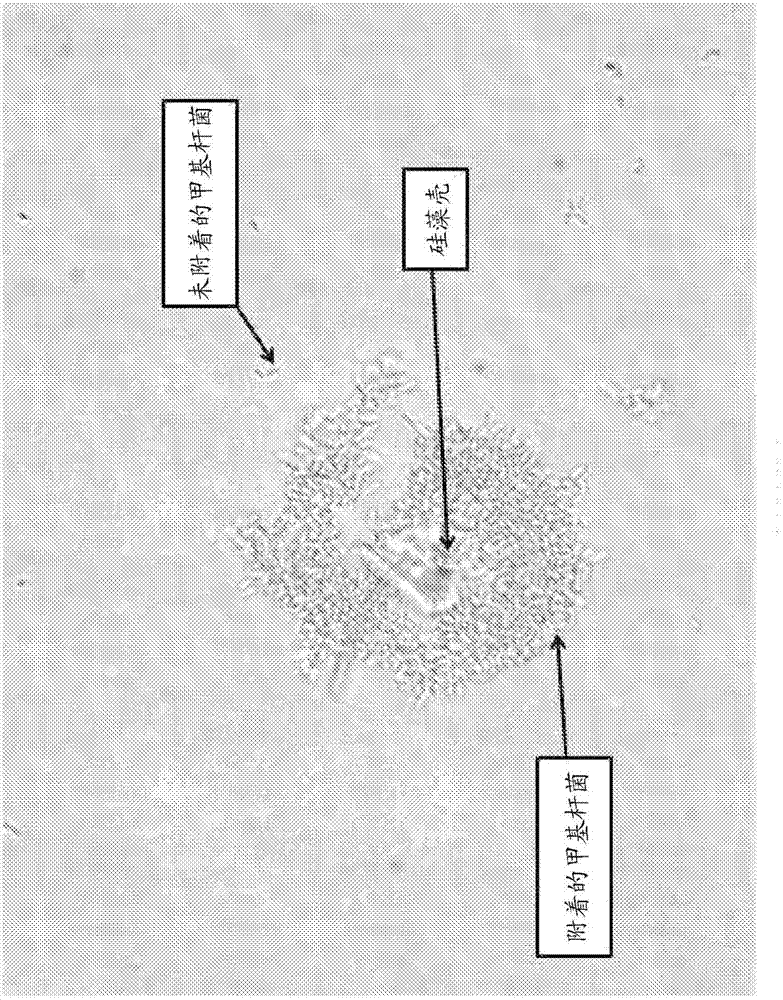
Microbial fermentation methods and compositions
A composition, fermentation product technology, applied in botanical equipment and methods, biofuels, immobilized on or in biological cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] The growth of embodiment 1.PPFM bacteria on solid agar plate medium.
[0111] Various standard media were tested for the growth of PPFM bacteria on solid agar plate media.
[0112] One medium used is ammonium mineral salts (AMS) medium (Whittenbury et al., 1970). AMS medium per liter contains 700 mg of anhydrous potassium dihydrogen phosphate, 540 mg of anhydrous potassium dihydrogen phosphate, 1 g of magnesium sulfate heptahydrate, 500 mg of anhydrous ammonium chloride, 200 mg of dehydrated calcium chloride, 4 mg of heptahydrate Ferric sulfate, 100 micrograms zinc sulfate heptahydrate, 30 micrograms manganese chloride tetrahydrate, 300 micrograms anhydrous boric acid, 200 micrograms cobalt chloride hexahydrate, 10 micrograms dehydrated copper chloride, 20 micrograms nickel chloride hexahydrate and 60 micrograms dehydrated Sodium molybdate.
[0113] Prepare AMS medium from the 4 stock solutions listed below.
[0114] Stock Solution I: For 1 L at 50X concentration
...
Embodiment 2
[0137] The growth of embodiment 2.PPFM bacteria in clarified single-phase liquid medium.
[0138] For those 4 solid agar plate media found in Example 1 to support the fastest and most abundant growth of the PPFM bacterium M. extorquens, corresponding liquid forms (that is, without addition of agar) were prepared and tested . These 4 liquid media prepared as described in Example 1 (with the only exception that they did not contain any agar) were all water-clear liquids with all components in solution. To a flask containing 100 ml of these 4 liquid media, add an inoculum of the PPFM bacterium M. extorquens to obtain approximately 1x10 5 Initial titer in colony forming units (CFU) / ml. The flasks were placed on a rotary shaking culture apparatus and incubated at 30°C and 250 rpm for 5 days. At the end of the 5 day incubation, determine the titer of PPFM bacteria in the flask. The result is:
[0139]
[0140] A surprising aspect of these results is that the nutrients presen...
Embodiment 3
[0141] Example 3.Growth of PPFM bacteria in a two-phase medium containing insoluble salt crystals.
[0142] To prepare biphasic media, liquid AMS+glycerol and peptone media are made turbid (ie given solid matter) by intentionally forming insoluble crystals of magnesium phosphate and / or calcium phosphate. In order to intentionally form insoluble crystals in the medium, the preparation method described in Example 1 was modified as follows. All components except trace metal stock solutions were mixed together prior to autoclaving. That is, to 940 ml of distilled water was added 20 ml each of stock solutions I, II and III, together with 10 g of glycerol and 10 g of peptone. After autoclaving, media preparation was completed by adding 1 ml of filter-sterilized trace metal stock solution. Autoclaving of the components of stock solutions I, II and III mixed together prior to autoclaving resulted in the formation of insoluble salt crystals, possibly predominantly magnesium hydrogen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com