Ketoconazole and clobetasol propionate cream and preparation method thereof
A technology of ketaxol cream and ketokang, which is applied to the field of ketocontazol cream and its preparation, can solve the problems of unsatisfactory skin penetration effect, bacterial or fungal infection, low bioavailability and the like, and achieves the promotion of local blood circulation, Increase the effect, enhance the effect of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment one: the preparation of ketocontasol emulsifiable cream of the present invention
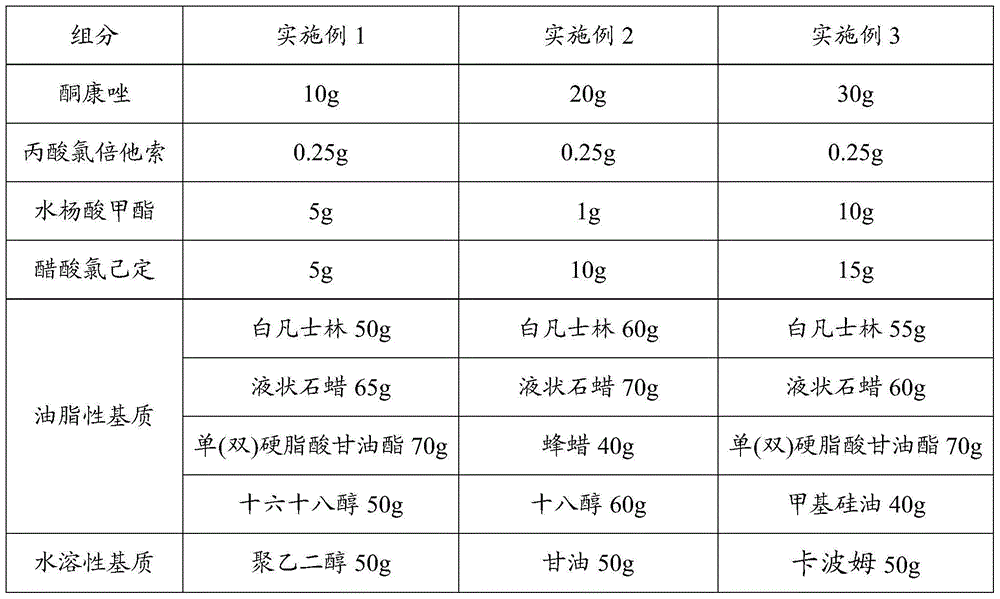
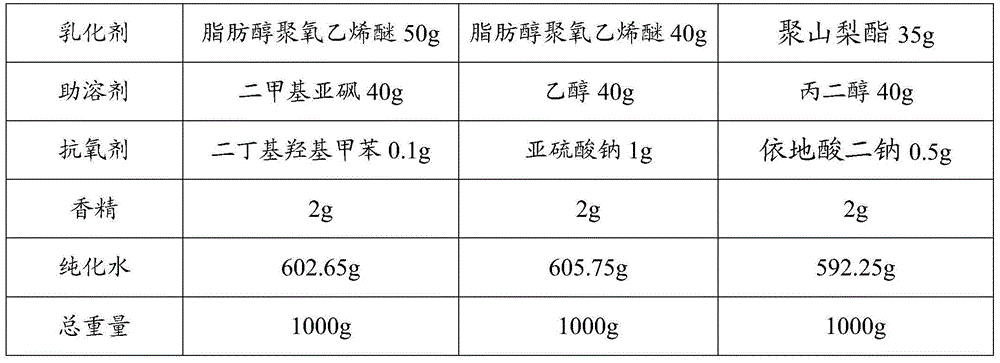
[0023] Weigh each component according to the formula in Table 1:
[0024] Table 1
[0025]
[0026]
[0027] Get the components in the formula shown in Table 1 respectively, according to the following steps, prepare the ketocontasol emulsifiable cream of the present invention:
[0028] 1) Take ketoconazole and clobetasol propionate, add them to the co-solvent, heat to 80±5°C, and stir to dissolve them completely;
[0029] 2) Take the oily base, heat it to 85±5°C, stir to melt it completely, keep it warm for 30 minutes, then cool it to 80±2°C, then add it into the solution obtained in step 1), and stir evenly to obtain the oil phase mixture;
[0030] 3) Take the water-soluble base, add chlorhexidine acetate, emulsifier, antioxidant and purified water, heat to 90±5°C, stir to dissolve it completely, keep it warm for 15 minutes, then cool to 80±2°C to obtain a water phase ...
Embodiment 2
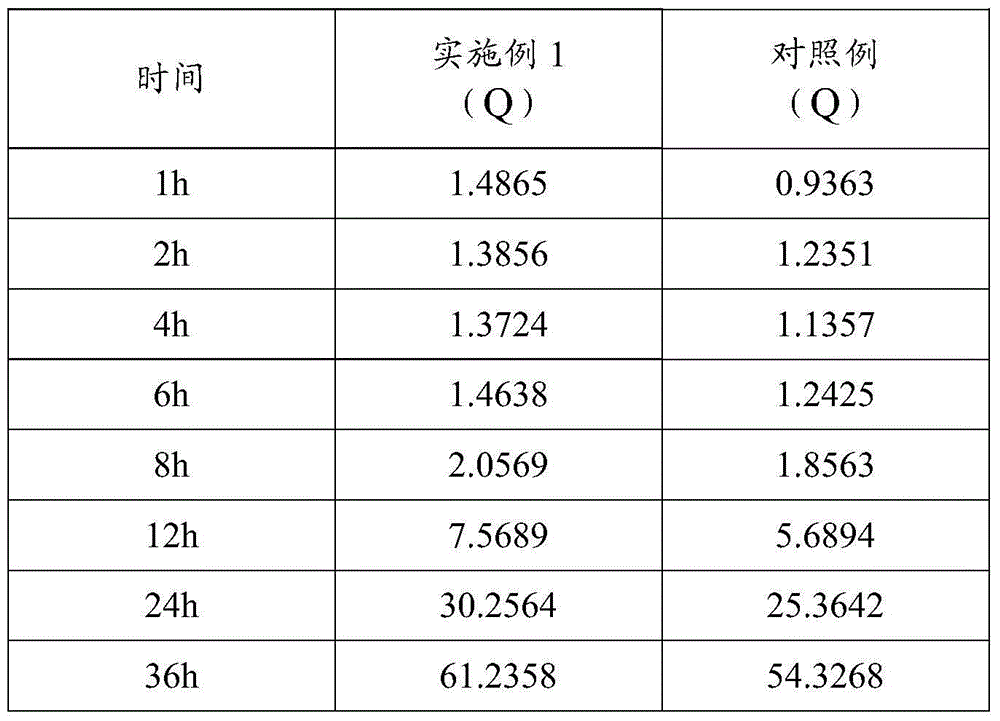
[0032] Embodiment 2: Determination of Transdermal Absorption Effect
[0033] 1. Preparation of Ketocontasol Cream Control Example
[0034] 1) Take 10g of ketoconazole and 0.25g of clobetasol propionate, add it to 40g of dimethyl sulfoxide, heat to 80±5°C, stir to dissolve completely;
[0035] 2) Take 50g of white petrolatum, 65g of liquid paraffin, 70g of mono (d) glyceryl stearate and 50g of cetostearyl alcohol, heat it to 85±5°C, stir it to completely melt, keep it warm for 30 minutes and then cool it down to 80±5°C 2°C, then added to the solution obtained in step 1), and stirred evenly to obtain an oil phase mixture;
[0036] 3) Take 50g of polyethylene glycol, add 5g of chlorhexidine acetate, 50g of fatty alcohol polyoxyethylene ether, 0.1g of dibutyl hydroxytoluene and 602.65g of purified water, heat to 90±5°C, stir to dissolve completely, Cool to 80±2°C after heat preservation for 15 minutes to obtain the aqueous phase mixture;
[0037] 4) Add the water phase mixture ...
Embodiment 3
[0043] Embodiment three: therapeutic effect measurement
[0044] 1. Case selection:
[0045] Take 114 outpatient cases of Dermatology and Venereology Department of the Third Affiliated Hospital of Sun Yat-sen Medical University.
[0046] Inclusion criteria: patients with skin diseases diagnosed with tinea corporis, tinea manuum and pedis, tinea versicolor, eczema, dermatitis, regardless of gender or age.
[0047] The following cases are not included in the observation objects: (1) pregnant and lactating women; (2) those who have received radiotherapy two weeks before treatment, systemic immunosuppressants or anti-bacterial, anti-viral and anti-helminth drugs; ( 3) Those who have used external antifungal drugs two weeks before treatment or those who have taken antifungal drugs internally one month before treatment; (4) Those who have a history of allergy to imidazoles; (5) Those who have liver, kidney or blood diseases.
[0048] 2. Treatment method
[0049] Wash the affected...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com