Spiro-condensed tricyclic spirooxazine photochromic compound and preparation method thereof
A photochromic, spirooxazine technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve problems such as increasing the cost of material synthesis, limiting the yield of target products, and inability to further improve, and achieve good light. The effect of chromogenic properties, good fatigue resistance and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
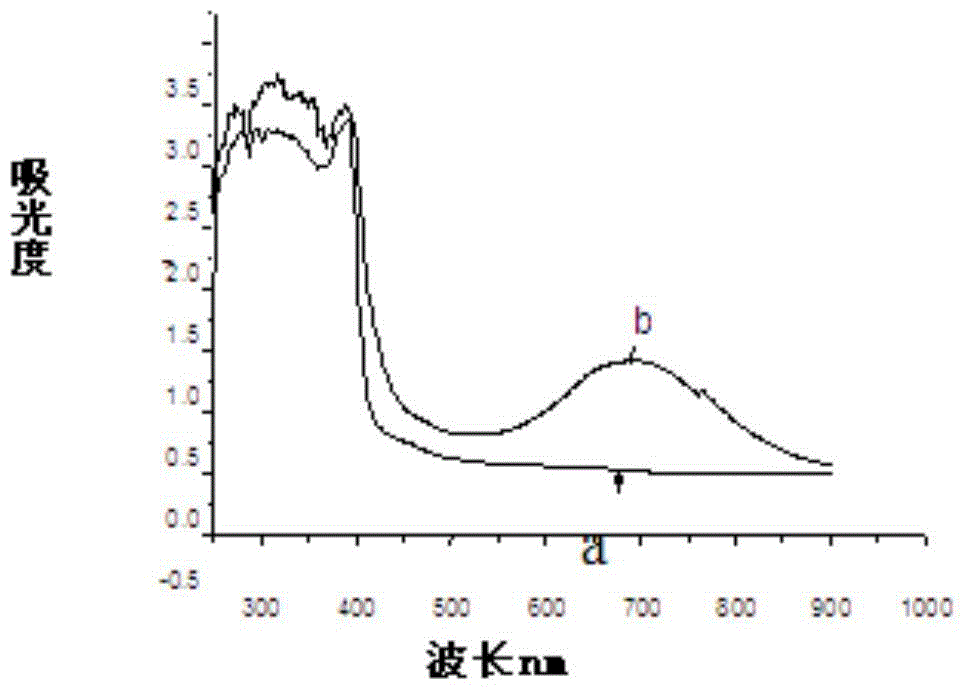
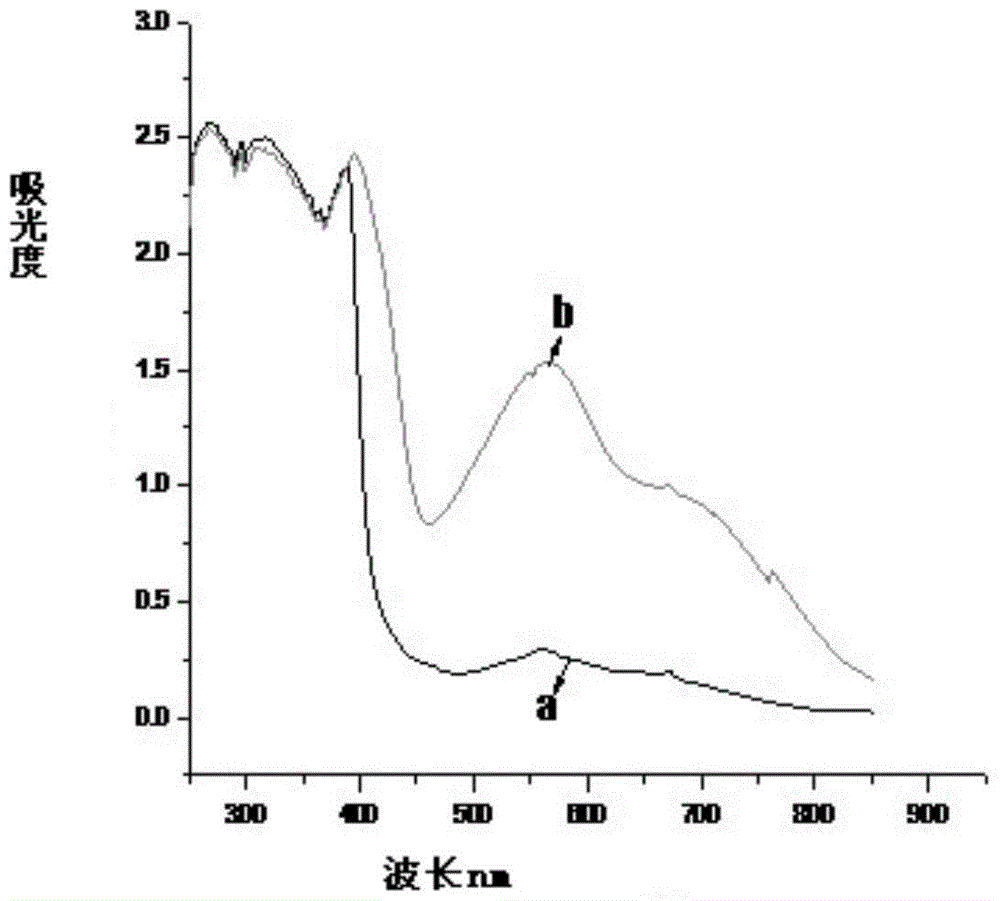
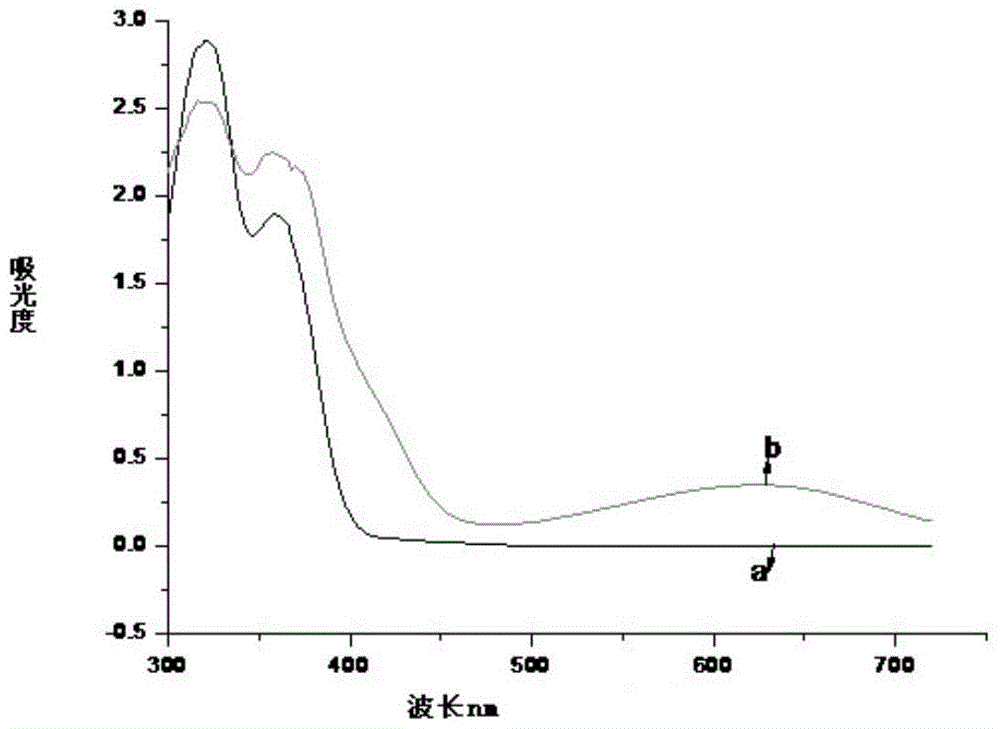
Image
Examples
preparation example Construction
[0042] Preparation of Intermediates:
[0043] Synthesis of intermediate 1--nitroso--2-naphthol copper complex (copper salt)
[0044] Under stirring, the dissolved 13.6g CuCl 2 400ml of aqueous solution was added dropwise to 800ml of a mixed solution of tetrahydrofuran and water dissolved with 39.8g of 1-nitroso-2-naphthol at a volume ratio of 1:1. After stirring for 20 minutes, vacuum filtration, washing with water, and drying gave 39.1 g of the product with a yield of 96%.
[0045] Synthesis of Intermediate Substituted 2,3,3--Trimethylindoline
[0046] In a 250ml three-necked flask, add 1.62mmol p-methoxyphenylhydrazine hydrochloride and 100ml glacial acetic acid, heat to reflux, slowly add 1.62mmol methyl isopropyl ketone dropwise, after the dropwise, continue to reflux for 10 hours. Stop the reaction, cool to room temperature with Na2 CO 3 The saturated solution was neutralized to neutral or slightly alkaline, extracted with dichloromethane, dried over anhydrous magnesi...
Embodiment 1
[0050] Target product: 5--chloro--1,2'--(1,2--ethyleneoxy)--1,3,3--trimethyl--6'--piperidinyl--spiro [2,3'--[3H]--naphtho[2,1--b][1,4]oxazine]
[0051] In a 250ml three-necked flask, add 1.0mmol of copper salt 1 and 4.0mmol of piperidine, use 50ml of anhydrous methanol as a solvent, and heat to reflux for 4h. in N 2 Under protection, 30 ml of anhydrous methanol solution containing 1.8 mmol 5-chloro N-(2-hydroxyethyl) 2,3,3-trimethylindoline bromide and 4 ml triethylamine were added dropwise, Continue the reflux reaction for 8h, TCL monitoring, stop the reaction and cool to room temperature, concentrate under reduced pressure, use petroleum ether / ethyl acetate (5:1) as eluent to carry out column chromatography to obtain a light yellow solid with a yield of 24 %.
[0052] m.p.226-228℃. 1 H NMR (400MHz, CDCl 3 )8.42(d, J=8.0Hz, 1H, Ar-H), 8.11(d, J=8.4Hz, 1H, Ar-H), 7.51-7.55(m, 1H, Ar-H), 7.40-7.44( m,1H Ar-H,),7.20(dd,J 1 =2.4Hz,J 2 =8.4Hz,1H,Ar-H),6.97(d,J=2.0Hz,1H,Ar-...
Embodiment 2
[0057] Target product: 5--chloro--1,2'--(1,2--ethyleneoxy)--1,3,3--trimethyl--6'--morpholinyl--spiro [2,3'--[3H]--naphtho[2,1--b][1,4]oxazine]
[0058] In a 250ml three-necked flask, add 1.0mmol of copper salt and 4.0mmol of morpholine, use 50ml of anhydrous methanol as a solvent, and heat to reflux for 4h. in N 2 Under protection, 30 ml of anhydrous methanol solution containing 1.8 mmol 5-chloro N-(2-hydroxyethyl) 2,3,3-trimethylindoline bromide and 4 ml triethylamine were added dropwise, The reflux reaction was continued for 8 h, TCL monitored until the reactant point disappeared, the reaction was stopped and cooled to room temperature, concentrated under reduced pressure, and column chromatography was performed using petroleum ether / ethyl acetate (3:1) as eluent to obtain a white solid. The yield was 20%. m.p.184-186℃ 1 H NMR (400MHz, CDCl 3 ) 8.17 (d, J = 8.4Hz, 1H, Ar-H), 7.63 (d, J = 8.4Hz, 1H, Ar-H), 7.44-7.47 (m, 1H, Ar-H), 7.32-7.36 ( m,1H,Ar-H),7.14(dd,J 1 =2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com