Mutant of maltogenic amylase and preparation method of mutant
A maltose amylase and mutant technology, applied in the fields of genetic engineering and enzyme engineering, can solve problems such as low efficiency, prolonged reaction time, and long production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: This example illustrates the preparation of wild maltogenic amylase.
[0019] (1) Construction of maltogenic amylase recombinant bacteria
[0020] According to the amyM amino acid sequence on NCBI (NCBI number: AAA22233.1), the amyM gene sequence on NCBI (NCBI number: M36539) was codon optimized, and the gene sequence amyM of maltose amylase was synthesized by chemical total synthesis. The plasmid used to construct the expression vector in E. coli is pET24a(+). The pET24a(+) plasmid and the plasmid with the amyM gene were digested with Nco Ⅰ and Hind Ⅲ, respectively. After the digested products were recovered by gel, they were ligated with T4 ligase overnight, and the ligated products were transformed into E. coli JM109 competent cells. The product was spread on an LB plate containing 100mg / L kanamycin, and cultured overnight at 37°C. Two single colonies were picked from the plate and inserted into LB liquid medium. After 8 hours, the plasmid was extracted f...
Embodiment 2
[0024] Example 2: This example illustrates the preparation of maltogenic amylase mutants.
[0025] (1) Site-directed mutation
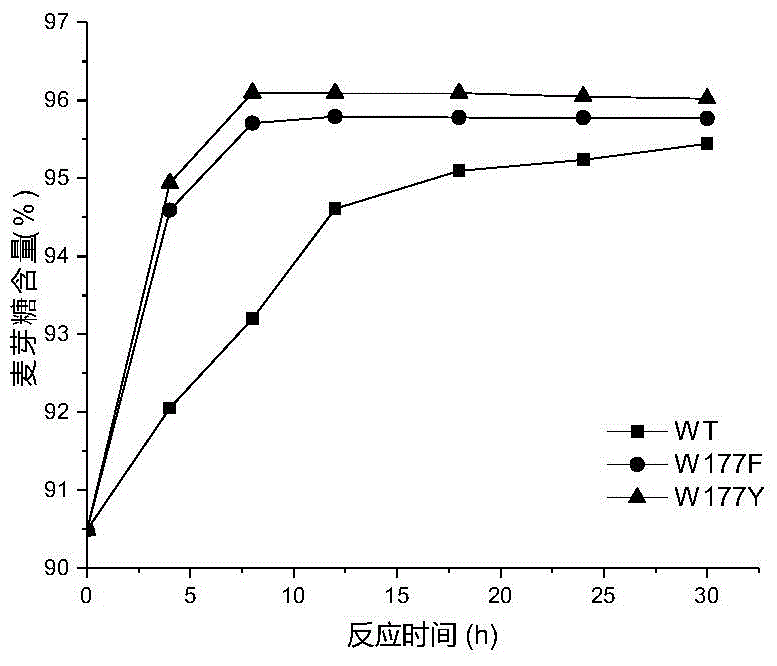
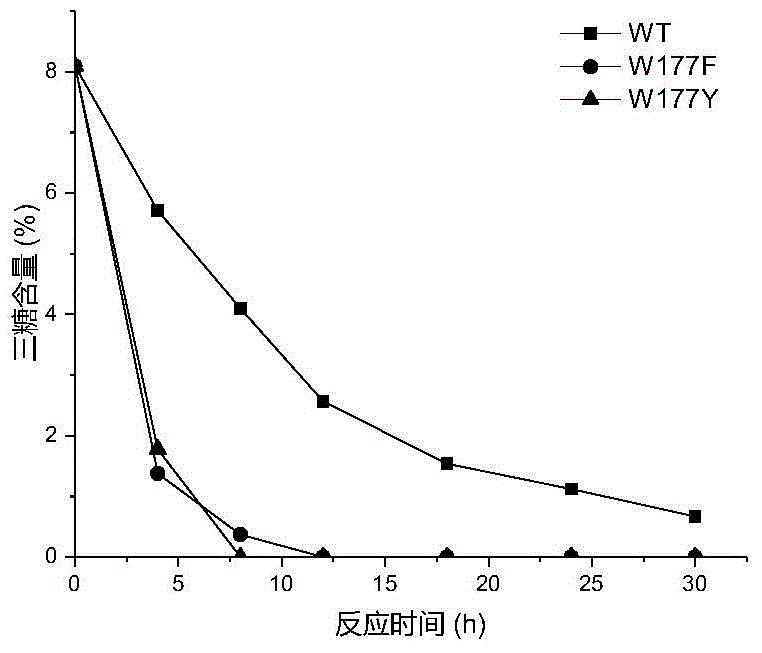
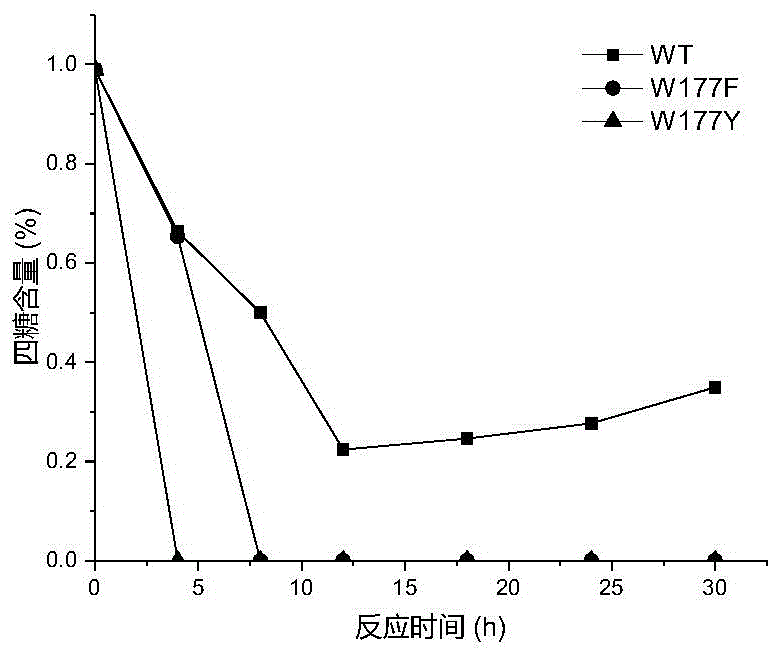
[0026] Based on the sequence alignment analysis of maltose amylase derived from B. stearothermophilus and cyclodextrin glucosyltransferase (CGTase) derived from Bacillus circulans strain 251, the protein structure of maltose amylase was simulated and analyzed , found the amino acid sites Trp177, Phe188, Ala229, Tyr258 that may be related to the transglycoside activity in maltose amylase. The 177th tryptophan (Trp) in the maltose amylase was mutated into histidine (His) and asparagine (Asn) respectively, which were marked as W177H, W177N; the 188th phenyl in the maltose amylase Alanine (Phe) was mutated into tyrosine (Tyr), marked as F188Y; the 229th alanine (Ala) in maltogenic amylase was mutated into valine (Val), threonine ( Thr) and leucine (Leu), respectively marked as A229V, A229T and A229L; the 258th tyrosine (Tyr) in maltose amylase was mutat...
Embodiment 3
[0054] Example 3: This example illustrates the enzyme activity assay of maltogenic amylase.
[0055] (1) Definition of enzyme activity unit
[0056] When using the 3,5-dinitrosalicylic acid method (DNS method) to measure the activity of maltose amylase, the amount of enzyme needed to catalyze the production of 1 μmol of reducing sugar per minute is taken as an activity unit.
[0057] (2) Enzyme Activity Determination Steps
[0058] Preheating: Take 2mL of 0.5% soluble starch solution (50mM pH5.5 citric acid buffer) in a test tube and place it in a 60°C water bath to preheat for 10min.
[0059] Reaction: Add 0.1mL sample enzyme solution, oscillate evenly, accurately time 10min, add 3mL DNS, oscillate evenly, put into ice water to terminate the reaction, boil in a boiling water bath for 7min. cool down.
[0060] Measurement: add distilled water to the above reaction system and adjust the volume to 15mL, and mix well. The absorbance was measured at a wavelength of 540nm and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Specific vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com