Controlled-release PLGA microsphere containing dexamethasone transforming growth factor and preparation method of controlled-release PLGA microsphere
A technology of transforming growth factor and dexamethasone, which is applied in the fields of medical science and prostheses, can solve the problems that cannot be realized, achieve good tissue compatibility, reduce local inflammatory response, and reduce inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
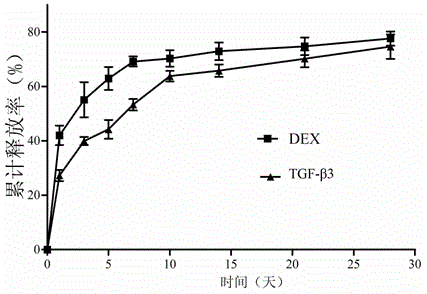
Image
Examples
Embodiment 1
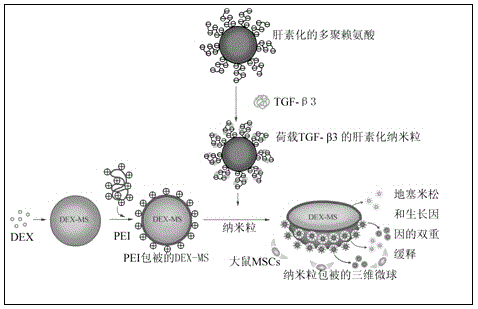
[0021] Example 1: Preparation of TGF-β3 / DEX / PLGA microspheres with 6gPLGA
[0022] (1) Preparation of DEX / PLGA microspheres by water-oil-water (w / o / w) latex method: Take PLGA (6g), DEX (60mg) and dissolve it in 30ml of dichloromethane, and use a glass syringe (specification, 20G) Inject the mixed solution into 300ml of an aqueous solution containing 2% (w / v) polyvinyl alcohol (poly(vinyl alcohol, PVA), stir with a magnetic stirrer at 600rpm for 2-3 hours (35°C), and remove di Chloromethane, collect the microspheres after centrifugation at 1500rpm for 2min, wash with distilled water for 6 times, and freeze-dry for subsequent use;
[0023] (2) Production of heparin-TGF-β3 nanoparticles
[0024] Take heparin solution (0.5 mg / ml) and poly-L-lysine (0.5 mg / ml), dissolve it in distilled water (PH 7.4) 1:1 to form a 0.5 mg / ml solution, take 100 ng / ml TGF-β3 and add to 0.5 mg / ml heparin / poly-L-lysine solution, stirred continuously for 15s, and incubated at room temperature for 30m...
Embodiment 2
[0027] Example 2 : Make TGF-β3 / DEX / PLGA microspheres with 12g PLGA.
[0028] (1) Preparation of DEX / PLGA microspheres
[0029] Take PLGA (12g), DEX (120mg) and dissolve it in 60ml of dichloromethane, use a glass syringe (specification, 20G) to inject the mixed solution into 600ml of aqueous solution containing 2% (w / v) PVA, stir with a magnetic stirrer at 600rpm After 2-3 hours (35°C), dichloromethane was removed, and the microspheres were collected by centrifugation at 1500 rpm for 2 minutes, washed with distilled water for 6 times, and then freeze-dried for later use;
[0030] (2) Production of heparin-TGF-β3 nanoparticles
[0031]Take heparin solution (0.5 mg / ml) and poly-L-lysine (0.5 mg / ml), dissolve it in distilled water (PH 7.4) 1:1 to form a 0.5 mg / ml solution, take 100 ng / ml TGF-β3 and add to 0.5 mg / ml heparin / poly-L-lysine solution, stirred continuously for 15s, and incubated at room temperature for 30min; a mixture with a molar charge ratio in the range of 0-3.1...
Embodiment 3
[0034] Example 3 : Make TGF-β3 / DEX / PLGA microspheres with 24g PLGA.
[0035] (1) Preparation of DEX / PLGA microspheres
[0036] Take PLGA (24g), DEX (240mg) and dissolve it in 240ml of dichloromethane, use a glass syringe (specification, 20G) to inject the mixed solution into 1200ml of an aqueous solution containing 2% (w / v) PVA, stir with a magnetic stirrer at 600rpm After 2-3 hours (35°C), dichloromethane was removed, and the microspheres were collected by centrifugation at 1500 rpm for 2 minutes, washed with distilled water for 6 times, and then freeze-dried for later use;
[0037] (2) Production of heparin-TGF-β3 nanoparticles
[0038] Take heparin solution (0.5 mg / ml) and poly-L-lysine (0.5 mg / ml), dissolve it in distilled water (PH 7.4) 1:1 to form a 0.5 mg / ml solution, take 100 ng / ml TGF-β3 and add to 0.5 mg / ml heparin / poly-L-lysine solution, kept stirring for 15 seconds, and incubated at room temperature for 30 minutes;
[0039] (3) Production of TGF-β3 / DEX / PLGA mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com