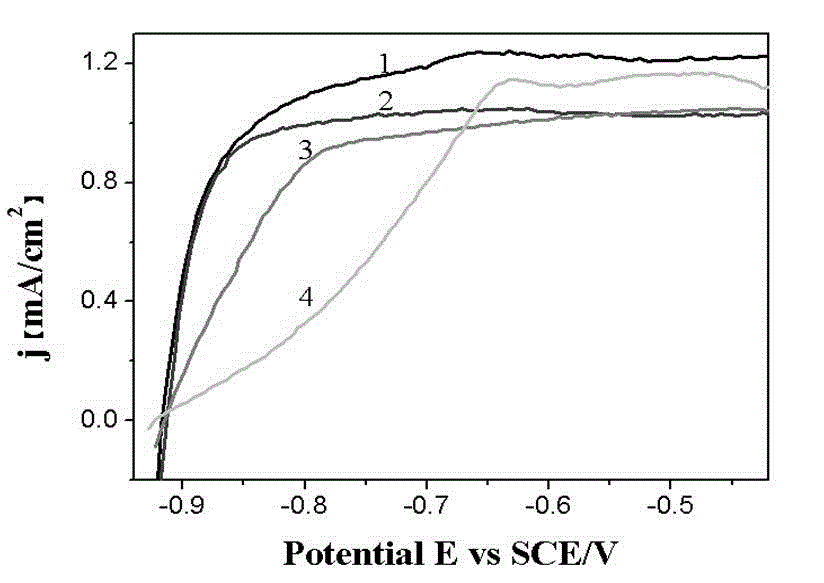
Carbon-loaded core-shell catalyst with nano copper nickel alloy core-precious metal shell and preparation method of catalyst
A core-shell catalyst, copper-nickel alloy technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., to avoid gradual corrosion and shedding, saving precious metals, alloying high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
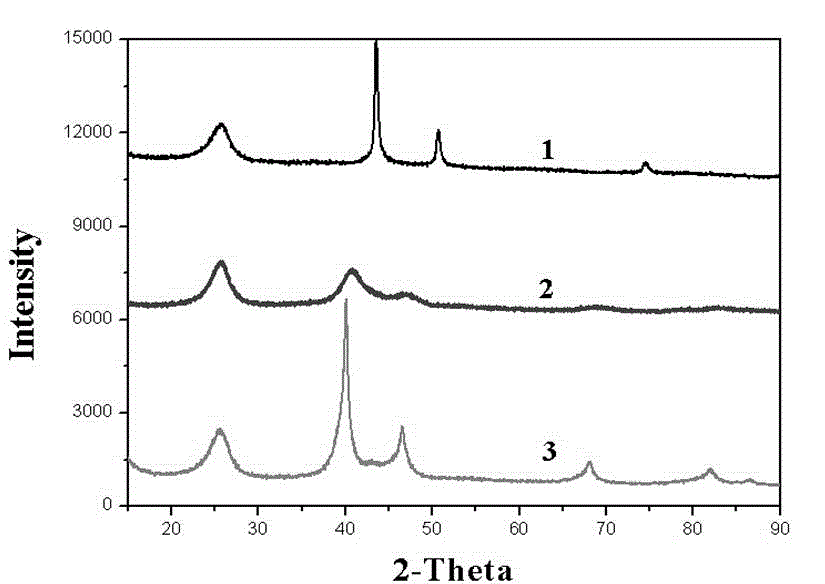
[0019] Add 400 mg of oxidized carbon powder to 40 mL of ethanol and water (volume ratio 1:1) mixed solvent, then add 100 mg of nickel nitrate hexahydrate and 303 mg of copper nitrate trihydrate. Stir at 60°C until the water and ethanol volatilize to form a paddle, then transfer to a vacuum drying oven and dry at 70°C for 4 hours to obtain CuNi salt / C. CuNi salt / C is ground into powder, placed in a porcelain boat and placed in an atmosphere tube furnace. 2 -Ar (volume flow rate ratio 20:80) reduction heat treatment at 500 °C for 1 h in a reducing atmosphere, and then naturally cooled to room temperature to obtain CuNi 4: 1 / C powder, its corresponding XRD spectrum is as figure 2 As shown in the middle curve 2, it is related to Ni 0.19 Cu 0.81 The one-to-one correspondence of the diffraction peaks of the alloy indicates that a good alloying effect has been obtained between nickel and copper. Weigh the above CuNi 4: 1 Add 450 mg of / C powder to 200 mL ethylene glycol...
Embodiment 2
[0021] Add 1000 mg of oxidized carbon powder to 100 mL of ethanol and water (volume ratio 1:1) mixed solvent, then add 248 mg of nickel nitrate hexahydrate and 760 mg of copper nitrate trihydrate. Stir at 50°C until the water and ethanol volatilize to form a paddle, then transfer to a vacuum drying oven and dry at 70°C for 10 hours to obtain CuNi salt / C. CuNi salt / C is ground into powder, placed in a porcelain boat and placed in an atmosphere tube furnace. 2 -Ar (volume flow rate ratio 20:80) reduction heat treatment at 600 °C for 1 h in a reducing atmosphere, and then naturally cooled to room temperature, the obtained CuNi with good alloying 4: 1 / C powder, wherein the mass fraction of copper and nickel is about 20%. Weigh the above CuNi 4: 1 Add 1000 mg of / C powder into 500 mL of ethylene glycol solution dissolved with 200 mg of chloroplatinic acid hexahydrate and 25 mg of polyvinylpyrrolidone (PVP protective agent), stir and raise the temperature to 125 °C and the...
Embodiment 3
[0023] Add 400 mg of oxidized carbon powder to 30 mL of ethanol and water (volume ratio 1:1) mixed solvent, then add 100 mg of nickel nitrate hexahydrate and 303 mg of copper nitrate trihydrate. Stir at 50°C until the water and ethanol volatilize to form a paddle, then transfer to a vacuum drying oven and dry at 70°C for 10 hours to obtain CuNi salt / C. CuNi salt / C is ground into powder, placed in a porcelain boat and placed in an atmosphere tube furnace. 2 -Ar (volume flow rate ratio 20:80) reduction heat treatment at 600 °C for 1 h in a reducing atmosphere, and then naturally cooled to room temperature, the obtained CuNi with good alloying 4: 1 / C powder, wherein the mass fraction of copper and nickel is about 20%. Weigh the above CuNi 4: 1 Add 450 mg of / C powder to 200 mL ethylene glycol solution dissolved with 60 mg palladium chloride, 60 mg potassium chloride and 10 mg polyvinylpyrrolidone (PVP protective agent), stir and heat up to 130°C and then reflux 1h. . ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com