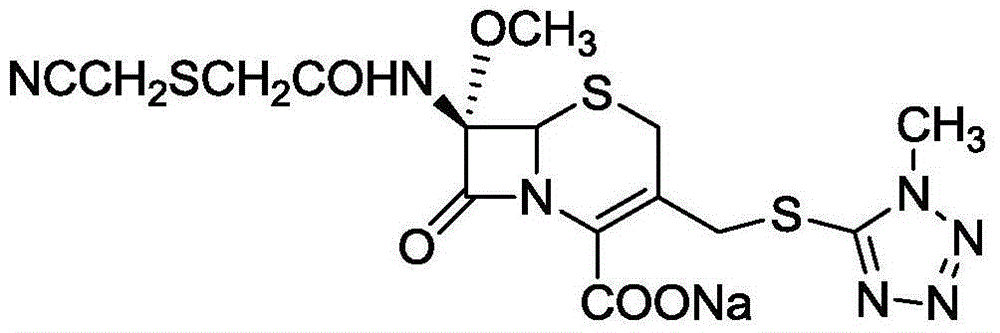
Preparation method for cefmetazole sodium
A technology for cefmetazole sodium and cefmetazole acid, which is applied in the field of preparation of cefmetazole sodium, can solve the problems of long production cycle, low product purity and high content of related substances, and achieves excellent product quality, high purity, and improved product quality. quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In a dry and clean three-necked flask, add 50g of 7-MAC and 600ml of chloroform, stir to dissolve, cool to -15°C, add 14g of pyridine, dropwise add 18g of cyanomethylmercaptoacetyl chloride, after the addition is complete, stir for 1 hour, add purified water: chlorine Sodium chloride: 40ml of a mixed solution of concentrated hydrochloric acid (9:1:2) was used to stop the reaction, and the layers were separated, and the organic phase was taken, dried, and concentrated to obtain 57.7g of cefmetazole diphenylmethyl ester, with a molar yield of 95%, HPLC 99.0% purity.
[0040] Cefmetazole diphenylmethyl ester purity test conditions (the same below): use octadecylsilane bonded silica gel as filler; use phosphate buffer (take 2.72g of potassium dihydrogen phosphate, add water to dissolve and dilute to 1000ml)- Acetonitrile (50:50) is the mobile phase; the detection wavelength is 214nm, and the resolution of the Mz-2 peak and the adjacent impurity peak should meet the requirem...
Embodiment 2
[0045] In a dry and clean three-neck flask, add 50g of 7-MAC, add 600ml of chloroform and stir to dissolve, cool to -15°C, add 10g of dimethylaminopyridine, dropwise add 18g of cyanomethylthioacetyl chloride, after the addition is complete, stir for 1 hour, add Purified water: 45ml of mixed solution of sodium chloride:concentrated hydrochloric acid (9:1:2) to stop the reaction, leave to stand and separate layers, take the organic phase, dry and concentrate to obtain 58.1g of cefmetazole diphenylmethyl ester, molar yield 95.7%, HPLC purity 99.3%.
[0046] Take 800ml of dichloromethane and cool down to 5-10°C, add 45g of aluminum trichloride under stirring, add 115g of anisole dropwise, control the temperature at 15-20°C, and cool down to -20~-25°C after adding; keep stirring and add cephalosporin 50g of methazole diphenylmethyl ester, react for 0.5 hours, add 50ml of a mixture of acetone:water:concentrated hydrochloric acid (weight ratio 8:8.2:1) cooled to below 0°C to terminat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com