Triazole-copper trifluoromethanesulfonate complex capable of catalyzing phenylboronic acid and preparation method of triazole-copper trifluoromethanesulfonate complex
A technology of copper complexes and m-benzenebistriazole copper, which is applied in the direction of copper organic compounds, organic compounds/hydrides/coordination complex catalysts, hydrocarbons, etc., can solve the problem of expensive palladium catalysts and achieve reaction operation Simple and easy to implement, low production cost and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
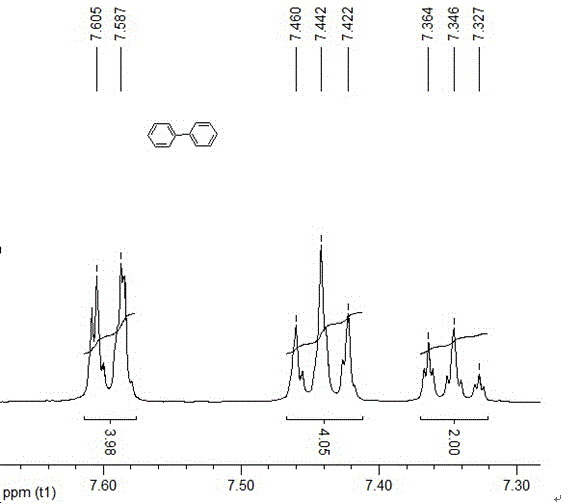
Examples
Embodiment 1
[0027] Preparation of 4-(3-(4H-1,2,4-triazol-4-yl)phenyl)-4H-1,2,4-triazole (L) Ligand
[0028] Add m-phenylenediamine (1 mmol) and bisformylhydrazide (2 mmol) respectively into a 50 mL three-neck round-bottom flask equipped with a magnet, reflux condenser and thermometer, and start stirring at 100 ° C for 12 hours. After the reaction was completed, the reaction solution was cooled to room temperature, and a large amount of precipitate was precipitated, which was recrystallized with water and ethanol, and the yield was 86%. Elemental Analysis C 10 h 8 N 6 Theoretical: C, 56.60; H, 3.80; N, 39.60. Experimental values: C, 56.56; H, 3.75; N, 39.56. The molar ratio of m-phenylenediamine to bisformylhydrazide is 1:2.
Embodiment 2
[0030] Cu(CF 3 SO 3 )2 and 4-(3-(4H-1,2,4-triazol-4-yl)phenyl)-4H-1,2,4-triazole) (L) in a molar ratio of 1:1;
[0031] L (0.0424 g, 0.2 mmol), Cu(CF 3 SO 3 ) 2 (0.0691 g, 0.2 mmol), H 2 O (6 mL), CH 3 CN (4 mL), water hot 100 o After three days in C, it was slowly lowered to room temperature. After opening the kettle, there are yellow rod-shaped crystals suitable for X-ray single crystal diffraction analysis. Yield: 35% (calculated based on L). Elemental analysis (C 33 h 26 Cu 3 f 9 N 18 o 10 S 3 ) Theoretical value (%): C, 30.67; H, 2.03; N, 19.51. Found: C, 30.69; H, 2.06; N, 19.59.
[0032] We also tried other ratios such as Cu(CF 3 SO 3 ) 2 If the molar ratio to L is 2:1, no crystalline compound can be obtained no matter how long the hydrothermal reaction time is. Therefore Cu(CF 3 SO 3 ) 2 The molar ratio of L and L is 1:1 is the best reaction ratio.
Embodiment 3
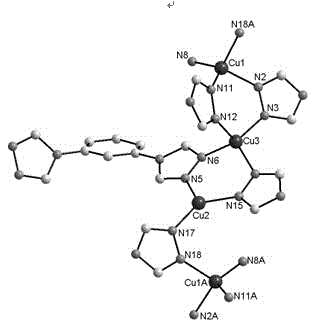
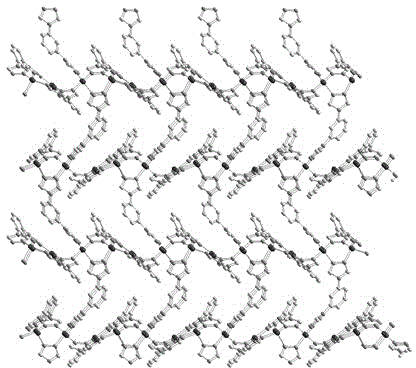
[0034] The crystal structure was determined using an APEX II CCD single crystal diffractometer, using graphite monochromatized Mokα rays (λ = 0.71073 ?) as the incident radiation, and ω -2 θ Diffraction points are collected by scanning, and the unit cell parameters are obtained by least square method correction. The crystal structure is solved by software from the difference Fourier electron density map, and corrected by Lorentz and polarization effects. All H atoms were synthesized by difference Fourier transform and determined by ideal position calculation. The detailed crystal determination data are shown in Table 1. Structural primitives see figure 1 , the three-dimensional structure of the complex see figure 2 .
[0035] Table 1. Complexes 1 The crystallographic data of
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com