Preparation and application of polyelectrolyte/silver halide nanocomposite antibacterial material
A technology of nanocomposites and polyelectrolytes, applied in applications, botany equipment and methods, animal repellants, etc., can solve the problems of long-acting antibacterial, easy loss, low rate, etc., and achieve great practical significance and Economic value, good alcohol solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
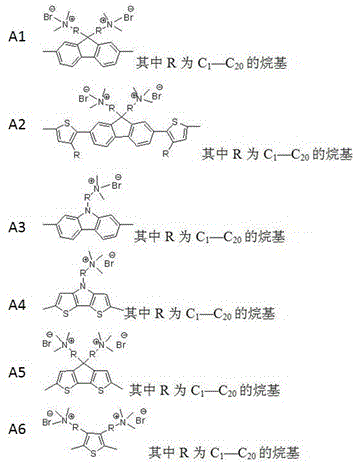
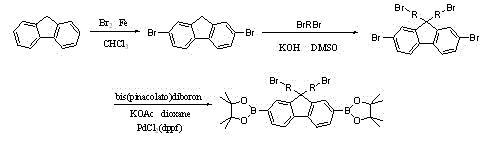
[0024] Example 1: Preparation of 9,9-disubstituted-2,7-diboronate fluorene
[0025] Take the preparation of 9,9-dibromohexyl-2,7-diboronate fluorene as an example to illustrate (see attached image 3 ):
[0026] Weigh fluorene (6g, 36mmol) and dissolve it in chloroform (40ml), add iron powder (32.5mg, 0.58mmol), cool in an ice bath, slowly add a mixture of liquid bromine (5ml) and chloroform (5ml), dropwise After completion, keep the low temperature reaction for 3 hours. After the reaction, add sodium bisulfite aqueous solution to remove excess bromine, extract with dichloromethane, wash the organic phase with water, dry over night with anhydrous sodium sulfate, evaporate the solvent, and then recrystallize with chloroform to obtain white crystal product 2, 7-Dibromofluorene.
[0027] Put 2,7-dibromofluorene (3.24g, 10mmol), tetrabutylammonium bromide (0.64g, 2mmol), dimethyl sulfoxide (50ml), potassium hydroxide aqueous solution (50ml, 50%) in three ports of 250ml A s...
Embodiment 2
[0029] Example 2: Preparation of N-substituted-3,6-diboronate carbazole
[0030] Take the preparation of 3,6-diboronate-N-bromohexylcarbazole as an example to illustrate (see the attached Figure 4 ):
[0031] Carbazole (12.54g, 75mmol), carbon disulfide (375ml) and anhydrous pyridine (24ml) were put into a reaction flask, cooled in an ice bath, and liquid bromine (28.30g, 177mmol) was slowly added dropwise. After the dropwise addition, the ice bath device was removed, the temperature was gradually raised to 15°C, and the mixture was stirred at 15°C for 2.5 hours. After the reaction, the reaction solution was poured into dilute hydrochloric acid, a pale yellow precipitate formed, filtered, washed with dilute sodium hydroxide solution 3 times, then washed with distilled water until neutral, and dried. Recrystallize with absolute ethanol and dry to obtain white needle-like crystals, which is the target product 3,6-dibromocarbazole.
[0032]Weigh 3,6-dibromocarbazole (5g, 15...
Embodiment 3
[0034] Example 3: Preparation of N-substituted-2,6-diboronate bithienopyrrole
[0035] Take the preparation of 2,6-diboronate-N-bromohexylbithienopyrrole as an example to illustrate (see the attached Figure 5 ):
[0036] Weigh 3,3'-dibromo-2,2'-bithiophene (2g, 6.18mmol), sodium tert-butylate (1.42g, 14.8mmol), Pd 2 dba 3 (0.142g, 0.155mmol) was dissolved in toluene (12ml). After stirring for 20 minutes, n-hexylamine bromide (1.11 g, 6.18 mmol) was added. Under the protection of argon, it was heated to 110°C and reacted for 7 hours. After the reaction, it was cooled to room temperature, and the organic phase was separated, extracted with dichloromethane, washed with water, and dried overnight with anhydrous sodium sulfate. The solvent was removed and separated by column chromatography to obtain the product N-bromohexylbithienopyrrole.
[0037] Weigh N-bromohexylbithienopyrrole (0.51g, 1.5mmol) and dissolve it in chloroform (20ml), add NBS (0.831g, 4.67mmol), and reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com