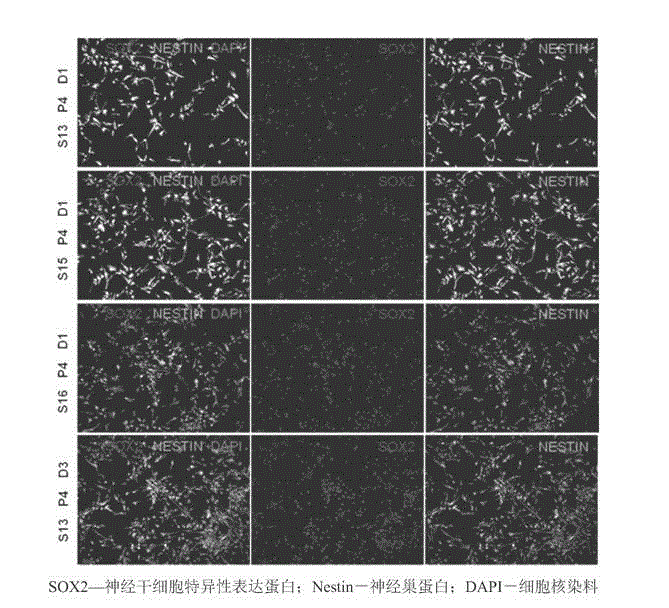
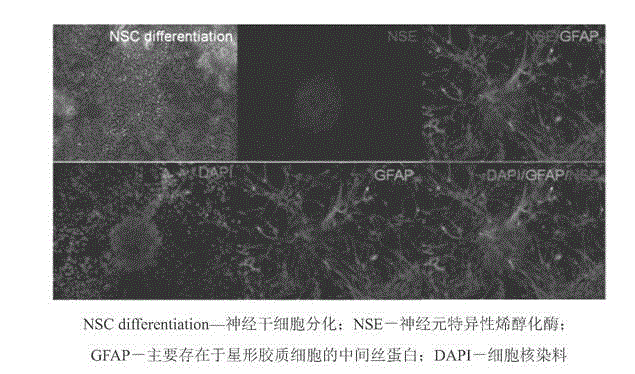
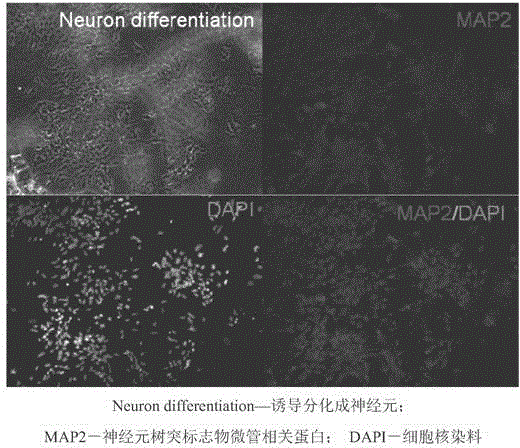
Clinical-grade serum-free medium for adherent culture of human neural stem cells
A serum-free medium and adherent culture technology, applied in the field of stem cell culture, to achieve the effects of protecting cells, improving utilization, and avoiding virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Comparison of neural stem cell adherence and suspension culture to clinical transformation.
[0042] The key to the clinical application of neural stem cells lies in the large-scale preparation of stem cells and the use of stem cells that meet the requirements for clinical use. According to the current literature reports, the proliferation of adherent cultured neural stem cells is faster and the adherent cells are easier to prepare in large scale.
[0043] The adherent culture cells (P3) and suspension culture cells (P3) were digested with Accutase enzyme, and the collected cells were frozen in liquid nitrogen with the same freezing medium and the same density for three months. Inoculated into coated culture plates to observe cell morphology. Results The survival rate of suspension cells was only about 60% after cryopreservation, while the survival rate of adherent cultured cells was over 90%. One day after the cells recovered and adhered to the wall, their...
Embodiment 2
[0044] Embodiment 2: Screening of each component in the serum-free medium supplement
[0045] By consulting all kinds of information, various additives that may be beneficial to cell proliferation are screened out, including the following, adenosine, transferrin, progesterone, putrescine, sodium selenite, apotransferrin, HSA, LIF, B-ME, VA, VC, Ve, Ve acetate, estradiol, thyroxine, ethanolamine, lipids, TGF beta, hydrocortisone, catalase, superoxide dismutase, glutathione , Triiodothyronine, L-carnitine, galactose, etc. Among them, the first five are N2 components. The present invention prepares them according to the concentration of each component in N2 as a basic additive, and then adds other various cytokines, BFGF, and EGF at appropriate concentrations therein, and cultivates NSCs from P0 to P3. Cell proliferation speed, cell morphology and cell proliferation ability after subculture were used as evaluation indicators, and the effects of each factor on cell proliferation ...
Embodiment 3
[0056] Example 3 The serum-free stem cell culture of the present invention that can be used in clinical culture of human neural stem cells, after quality inspection, all indicators are up to standard, see Table 4:
[0057] Table 4: Detection items and results of human neural stem cell serum-free medium of the present invention
[0058]
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com