Drug composition for treating digestive system diseases
A digestive system disease and composition technology, applied in the field of medicine, can solve the problems that the dissolution rate of lansoprazole capsules cannot be better improved, the product yield and purity cannot reach the ideal effect, and it is not suitable for large-scale mechanized production. , to achieve good stability and impurity content, simple preparation method, and prevent damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
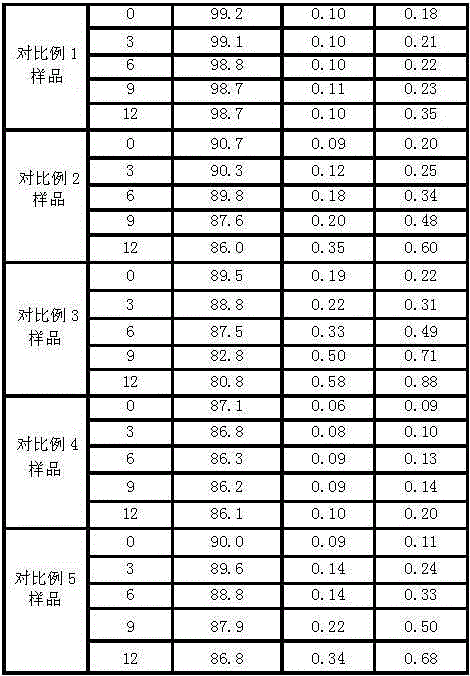
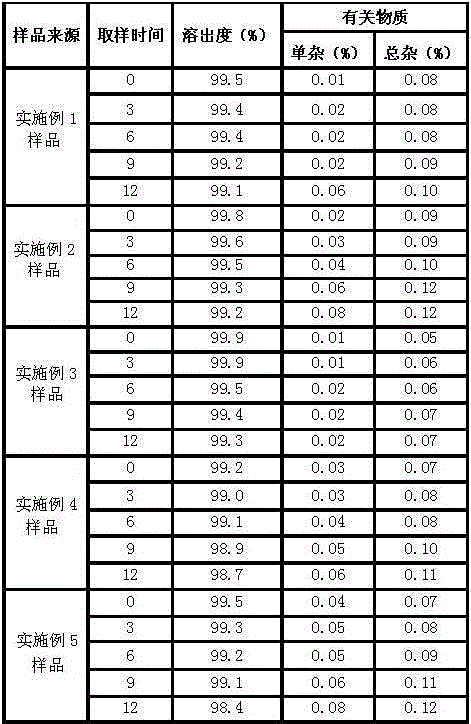
Image
Examples
Embodiment 1
[0040] Example 1: Lansoprazole enteric-coated capsules of the present invention are prepared
[0041] (1) Ball core prescription (parts by weight): Lansoprazole 8, starch 15, sucrose 8, hydroxypropyl cellulose 1, potassium sodium tartrate 1.8, carboxymethyl cellulose sodium 2, sodium lauryl sulfate 0.4, Povidone K30 2, 95% ethanol 25.
[0042] (2) Prescription of enteric coating solution (parts by weight): 5.6% of hypromellose phthalate, 1% of n-butyl stearate, 1% of talcum powder, and 140% of 95% ethanol.
[0043] (3) Preparation method:
[0044] The preparation of the enteric-coated capsules of the present invention can be obtained by methods commonly used in the art, including but not limited to the following preparation methods:
[0045] 1) Preparation of ball core
[0046] Lansoprazole, sucrose, potassium sodium tartrate, starch, hydroxypropyl cellulose, sodium carboxymethyl cellulose, and sodium lauryl sulfate are pulverized and mixed uniformly; use povidone K30 etha...
Embodiment 2
[0051] Example 2: Lansoprazole enteric-coated capsules of the present invention are prepared
[0052] (1) Ball core prescription (parts by weight): Lansoprazole 9, starch 16.4, sucrose 9.4, hydroxypropyl cellulose 1.5, potassium sodium tartrate 2.0, carboxymethylcellulose sodium 2.1, sodium lauryl sulfate 0.5, Povidone K30 2.4, 95% ethanol 26.1.
[0053] (2) Enteric coating solution prescription (parts by weight): Hypromellose phthalate 5.9, n-butyl stearate 1.25, talcum powder 1.1, 95% ethanol 145.
[0054] (3) Preparation method:
[0055] The preparation of the enteric-coated capsules of the present invention can be obtained by methods commonly used in the art, including but not limited to the following preparation methods:
[0056] 1) Preparation of ball core
[0057] Lansoprazole, sucrose, potassium sodium tartrate, starch, hydroxypropyl cellulose, sodium carboxymethyl cellulose, and sodium lauryl sulfate are pulverized and mixed uniformly; use povidone K30 ethanol so...
Embodiment 3
[0062] Example 3: Lansoprazole enteric-coated capsules of the present invention are prepared
[0063] (1) Ball core prescription (parts by weight): Lansoprazole 10, starch 21, sucrose 12, hydroxypropyl cellulose 2, potassium sodium tartrate 2.4, carboxymethyl cellulose sodium 3, sodium lauryl sulfate 0.6, Povidone K30 3, 95% ethanol 27.
[0064] (2) Enteric coating solution prescription (parts by weight): Hypromellose phthalate 7.3, n-butyl stearate 1.3, talcum powder 1.5, 95% ethanol 150.
[0065] (3) Preparation method:
[0066] The preparation of the enteric-coated capsules of the present invention can be obtained by methods commonly used in the art, including but not limited to the following preparation methods:
[0067] 1) Preparation of ball core
[0068] Lansoprazole, sucrose, potassium sodium tartrate, starch, hydroxypropyl cellulose, sodium carboxymethyl cellulose, and sodium lauryl sulfate are pulverized and mixed uniformly; use povidone K30 ethanol solution as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com