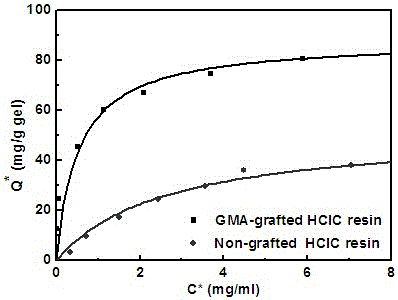
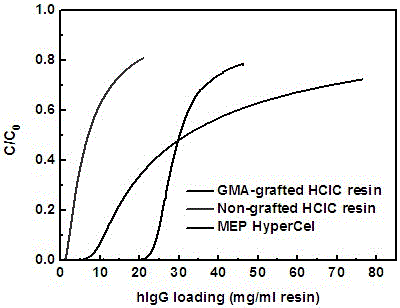
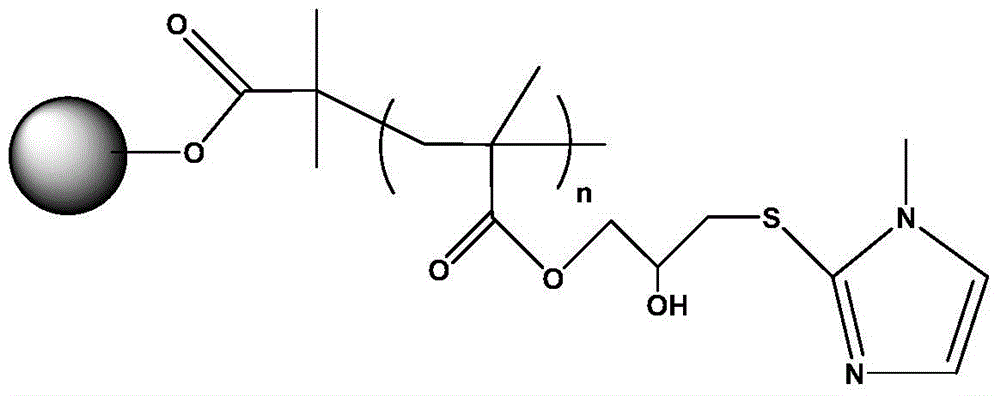
A polymer-grafted hydrophobic charge-induced chromatography medium and its preparation method
A hydrophobic charge and induction layer technology, applied in the field of protein chromatography separation technology, can solve problems such as no reports, and achieve the effects of high effective diffusion coefficient, convenient elution and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Take 10 g of drained agarose gel, replace the contained water with acetone, then add 100 ml of anhydrous tetrahydrofuran, 5 ml of 2-bromoisobutyryl bromide, 5.5 ml of anhydrous triethylamine and 0.25 g of 4-dimethylaminopyridine, 0 Under ice bath for 2h at 30°C, then activate with magnetic stirring in a sealed three-neck flask for 12 hours at 30°C, filter with suction, wash with acetone and deionized water to obtain the activated matrix; then mix the activated matrix, 2ml glycidyl methacrylate, 100ml80% Mix isopropanol solution (v / v), 0.03g copper bromide, 0.06g ascorbic acid, and 0.06ml pentamethyldiethylenetriamine for surface-initiated free radical polymerization, and magnetically stir in a sealed three-necked flask at 50°C React for 12 hours, filter with suction, wash alternately with acetone and deionized water, remove the copolymer, and obtain a polymer-grafted matrix; then mix the grafted matrix with 0.8g 2-mercapto-1-methylimidazole and 1M sodium carbonate soluti...
Embodiment 2
[0031] Take 10 g of drained agarose gel, replace the contained water with acetone, add 100 ml of anhydrous tetrahydrofuran, 1 ml of 2-bromoisobutyryl bromide, 1.1 ml of anhydrous triethylamine and 0.05 g of 4-dimethylaminopyridine, 0 °C Under ice bath for 2h, then magnetically stirred and activated in a sealed three-neck flask at 30°C for 12 hours, suction filtered, washed with acetone and deionized water to obtain the activated matrix; then the activated matrix, 0.5ml glycidyl methacrylate, 100ml 80 % isopropanol solution (v / v), 0.01g of copper bromide, 0.02g of ascorbic acid and 0.02ml of pentamethyldiethylenetriamine were mixed to carry out surface-induced free radical polymerization, and magnetically Stir the reaction for 12 hours, filter with suction, and wash alternately with acetone and deionized water to remove the copolymer to obtain a polymer-grafted matrix; then mix the grafted matrix with 0.2g 2-mercapto-1-methylimidazole and 1M sodium carbonate Buffer solution (pH...
Embodiment 3
[0033] Take 10 g of drained agarose gel, replace the contained water with acetone, add 200 ml of anhydrous tetrahydrofuran, 1 ml of 2-bromoisobutyryl bromide, 1.1 ml of anhydrous triethylamine and 0.05 g of 4-dimethylaminopyridine, 0 °C Under ice bath for 3h, then magnetically stirred and activated in a sealed three-necked flask at 30°C for 24 hours, suction filtered, washed with acetone and deionized water to obtain the activated matrix; then the activated matrix, 2ml glycidyl methacrylate, 100ml 80% Mix isopropanol solution (v / v), 0.03g copper bromide, 0.06g ascorbic acid, and 0.06ml pentamethyldiethylenetriamine for surface-initiated free radical polymerization, and magnetically stir in a sealed three-necked flask at 50°C React for 12 hours, filter with suction, wash alternately with acetone and deionized water, remove the copolymer, and obtain a polymer-grafted matrix; then mix the grafted matrix with 0.8g 2-mercapto-1-methylimidazole and 1M sodium carbonate solution (pH 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com