Polyimide and molded body thereof
A technology of polyimide and polyimide film, applied in the direction of coating, etc., can solve the problems of low hygroscopic solvent solubility, undiscovered polyimide, low linear thermal expansion coefficient, etc., and achieve high solution stability, The effect of excellent transparency and low linear thermal expansion coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] Regarding the preparation method of the polyimide of the present invention, taking the polyimide containing the structural unit shown in the following formula (2) when all R in the above formula (1) is a methyl group as an example, further Detailed explanation. In addition, even when R is another alkyl group or a hydrogen atom, it can still be prepared in the same way as polyimide containing the structural unit represented by formula (2).
[0091] Formula (2)
[0092]
[0093] The polyimide represented by the above formula (2) can be produced from tetracarboxylic dianhydride represented by the following formula (9).
[0094] Formula (9)
[0095]
[0096] The tetracarboxylic dianhydride shown in the above formula (9) can use diols shown in the following formula (10), that is, 2,2',3,3',5,5'-hexamethyl-biphenyl-4, 4'-diol (hereinafter sometimes abbreviated as HM44BP) or its diacetate body is prepared by a known esterification reaction with trimellitic acid.
[0...
Embodiment
[0143] The following examples illustrate the present invention, but are not limited to these examples. In addition, the physical property value in the following example was measured by the following method.
[0144] (Evaluation method)
[0145] The material property values and the like described in this specification are obtained by the following evaluation methods.
[0146]
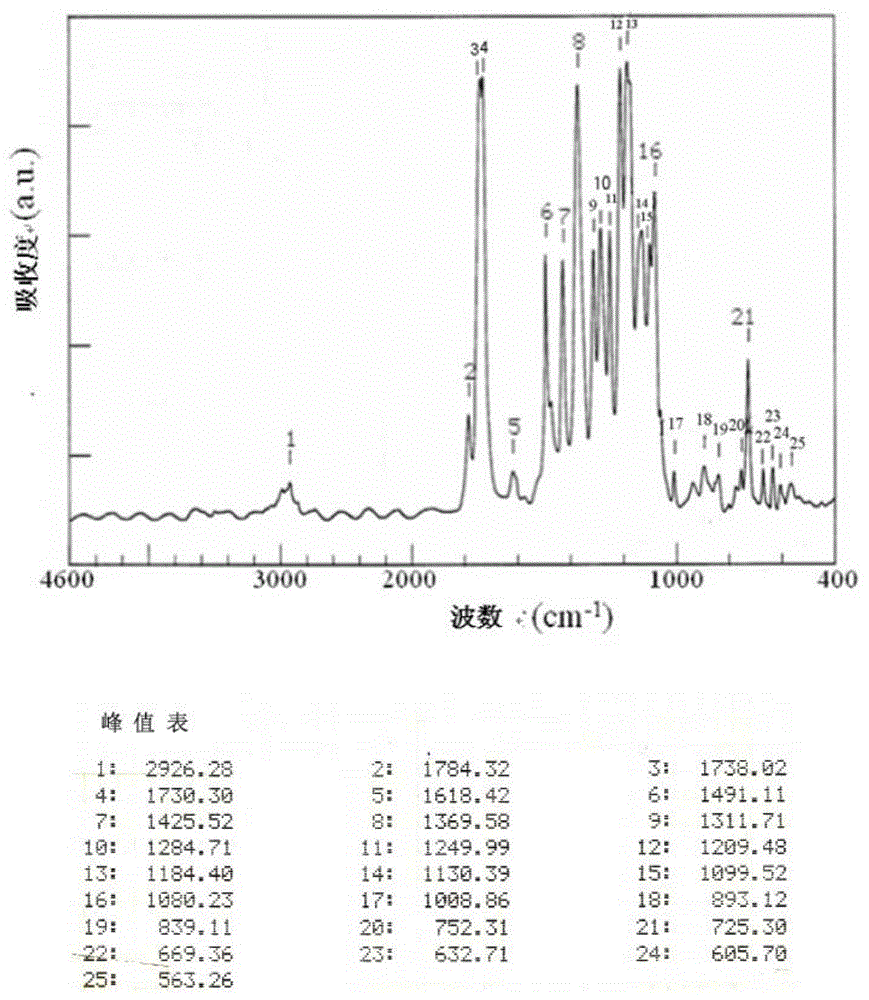
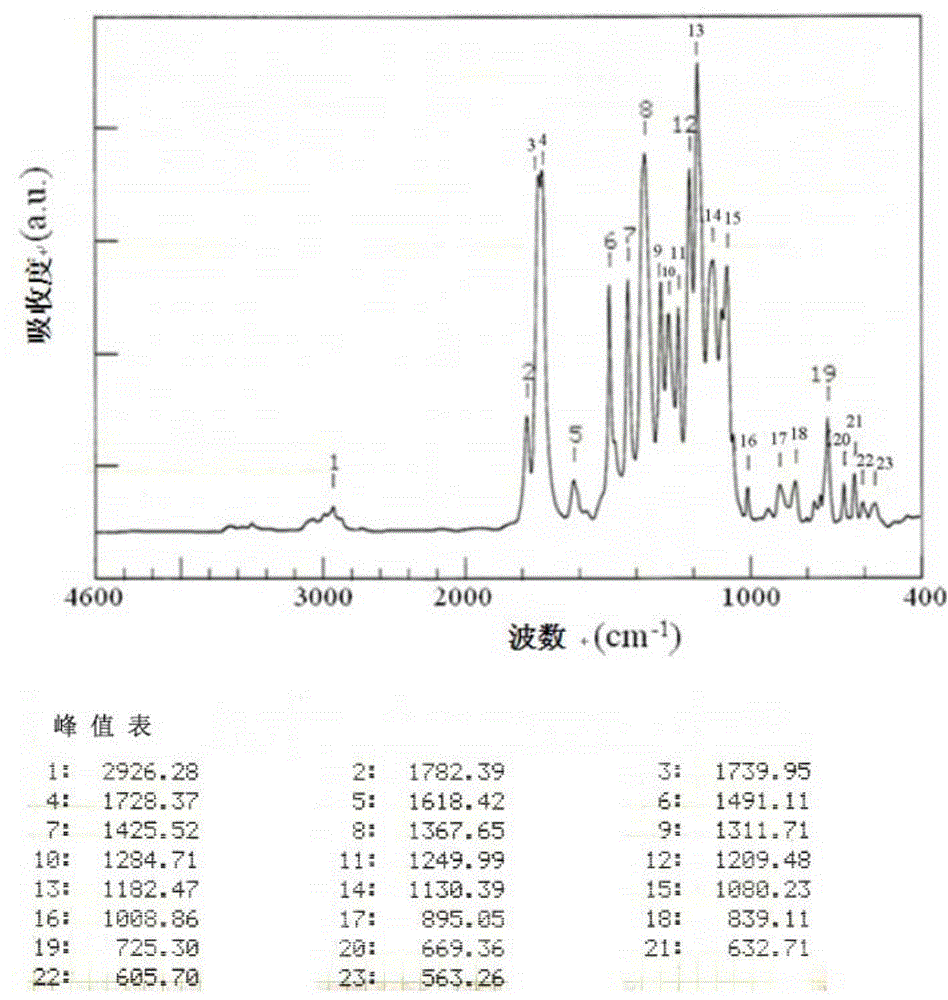
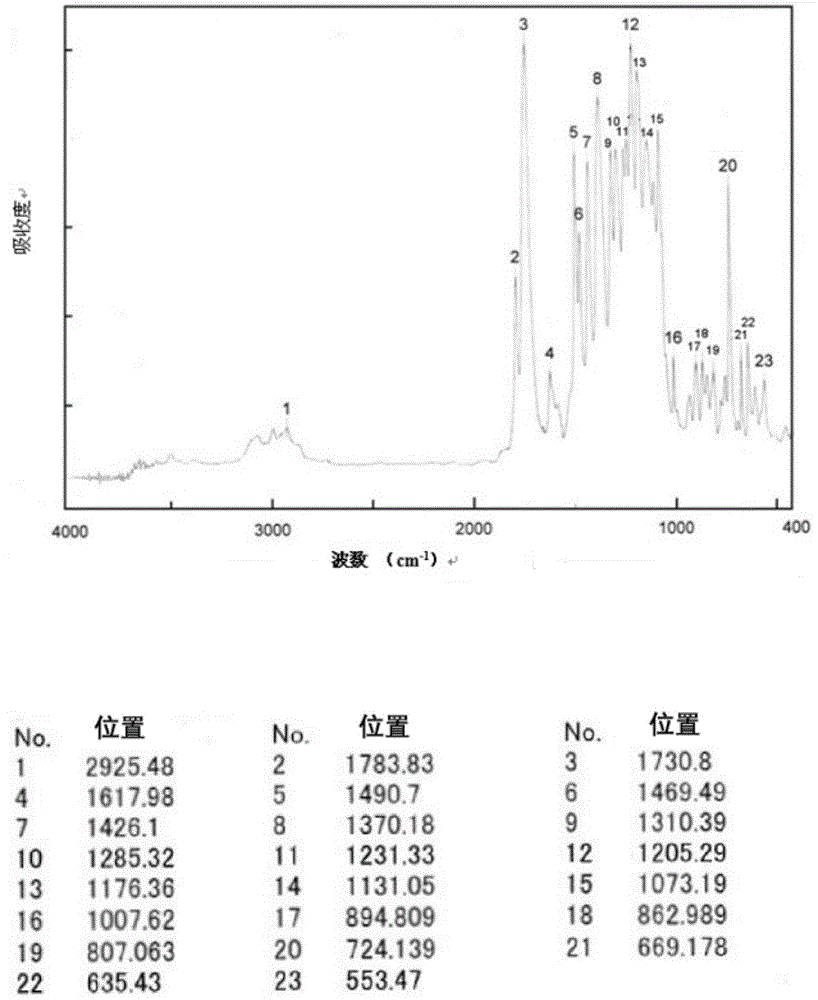
[0147] The infrared absorption spectrum of tetracarboxylic dianhydride was measured by the KBr method using Fourier transform infrared spectrophotometer FT / IR350 (made by JASCO Corporation). In addition, the infrared absorption spectrum of polyimide was measured by preparing a thin film sample (about 5 micrometers thick).
[0148] 1 H-NMR spectrum>
[0149] Using Fourier transform nuclear magnetic resonance JNM-ECP400 (manufactured by JEOL), measure tetracarboxylic dianhydride in deuterated dimethyl sulfoxide and chemically imidized polyimide powder. 1 H-NMR spectrum. Tetramethylsilane was used ...
Synthetic example 1
[0175] Synthesis of tetraformic dianhydride shown in formula (9)
[0176] (synthesis)
[0177] The synthesis|combination of tetracarboxylic dianhydride (TAHMBP) represented by formula (9) is as follows. 8.8493 g (42.0 mmol) of trimellitic anhydride was added to an eggplant-shaped flask, dissolved in 30 mL of dehydrated tetrahydrofuran (THF) at room temperature, and a septum was sealed to prepare solution A (solute concentration: 24.9% by weight). Then, in another flask, 5.4044g (20.0mmol) of 2,2',3,3',5,5'-hexamethyl-biphenyl-4,4'-diol (HM44BP) was dissolved at room temperature Solution B was prepared by adding 3.9 mL (48 mmol) of pyridine to 38 mL of dehydrated THF (solute concentration: 14.5% by weight), and sealing the septum.
[0178] Cool in an ice bath, slowly add solution B to solution A dropwise with a syringe while stirring, and then stir at room temperature for 24 hours. After the reaction, the white precipitate was filtered off and washed with THF and deionized w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com