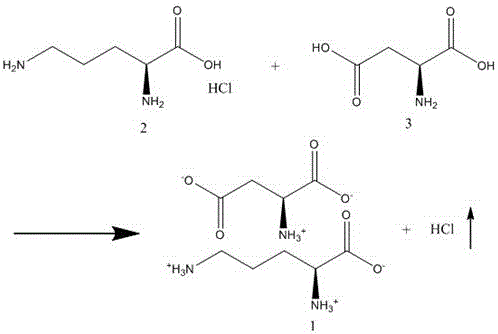
A kind of preparation method of aspartic acid ornithine
A technology of ornithine aspartate and ornithine aspartate is applied in the field of preparation of ornithine aspartate, and can solve the problems of not very huge difference in properties, low purity, difficult recycling of waste liquid, and the like, To achieve the effect of relatively low solvent toxicity, simple post-processing process, and reduction of human injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add ornithine hydrochloride (168.62g, 1mol) and aspartic acid (133.10g, 1mol) into the reaction kettle, add 603ml of water, raise the temperature to 30°C, start vacuuming with a water vacuum pump, and keep the pressure below 4000Pa During this period, water is continuously added to keep the liquid level of the reaction liquid near the initial position. As the vacuuming progresses, the internal pressure continues to decrease. After 1 hour of vacuuming, the internal pressure reaches about 3400Pa. Use a nitrate test solution to detect the content of chlorine ions in the extracted gas dissolved in water every 30 minutes. When the extracted gas has no chlorine , the reaction ends.
[0038] The reaction solution was added dropwise to 4.5L of methanol, and a large amount of precipitate was formed. After the dropwise addition was completed, it was stirred for 30 minutes, and filtered with suction to obtain the crude product of aspartic acid ornithine (260g), with a yield of abo...
Embodiment 2
[0041] Add ornithine hydrochloride (168.62g, 1mol) and aspartic acid (133.10g, 1mol) into the reaction kettle, add 900ml of ethanol, heat up to 50°C, start vacuuming with a water vacuum pump, and keep the pressure below 2000Pa During this period, ethanol was continuously added to keep the liquid level of the reaction solution near the initial position. As the vacuuming progresses, the internal pressure continues to decrease. After 1 hour of vacuuming, the internal pressure reaches about 1400Pa. Use a nitrate test solution to detect the content of chlorine ions in the extracted gas dissolved in water every 30 minutes. When the extracted gas has no chlorine , the reaction ends.
[0042] After the reaction, a large amount of precipitates were formed in the reaction solution, and the crude product of ornithine aspartate (258 g) was obtained by suction filtration, with a yield of about 97.2%.
[0043] Dissolve 258g of ornithine aspartic acid crude product in 500ml of water, add it d...
Embodiment 3
[0045] Add ornithine hydrochloride (168.62g, 1mol) and aspartic acid (133.10g, 1mol) into the reaction kettle, add 700ml of water, raise the temperature to 80°C, start vacuuming with a water vacuum pump, and keep the pressure below 500Pa During this period, water is continuously added to keep the liquid level of the reaction liquid near the initial position. As the vacuuming progresses, the internal pressure continues to drop. After 1 hour of vacuuming, the internal pressure reaches about 10Pa. Use a nitrate test solution to detect the content of chlorine ions in the extracted gas dissolved in water every 30 minutes. When the extracted gas has no chlorine , the reaction ends.
[0046] The reaction solution was added dropwise to 4.5L ethanol, a large amount of precipitate was formed, after the dropwise addition was completed, it was stirred for 30 minutes, and filtered with suction to obtain the crude product of aspartic acid ornithine (260g), with a yield of about 98%.
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com