Gold nanoparticle complex with effect of inhibiting nerve cell apoptosis and application thereof
A technology of nerve cell apoptosis and gold nanoparticles, applied in the gold nanoparticle complex and its application field, can solve the problems of no discovery, and achieve the effect of small size, easy degradation and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation of a gold nanoparticle complex capable of inhibiting nerve cell apoptosis
[0057] 1. Preparation of dithiodisuccinimidyl propionate (DTSP):
[0058] Dissolve 4.2504 g (0.02 mol) of 3,3'-dithiodipropionic acid (DTPA) and 4.6036 (0.04 mol) of N-hydroxysuccinimide (HOSu) in 20 mL of dimethyl Formamide; Dissolve 4.1266 g (0.02 mmol) of dicyclohexylcarbodiimide in 20 mL of dimethylformamide; add it dropwise to the reaction mixture under stirring, react in an ice bath for 24 hours, and filter; then Dilute with ethyl acetate, add dilute hydrochloric acid to remove the remaining dicyclic urea. Then the solvent was removed by evaporation to obtain a solid product, which was washed three to five times with dilute hydrochloric acid, then ethyl acetate was added to remove the aqueous hydrochloric acid solution, and the solvent was removed by rotary evaporation to obtain the product, namely DTSP.
[0059] 2. Preparation of thiolated chitosan (TCTS):
[00...
Embodiment 2
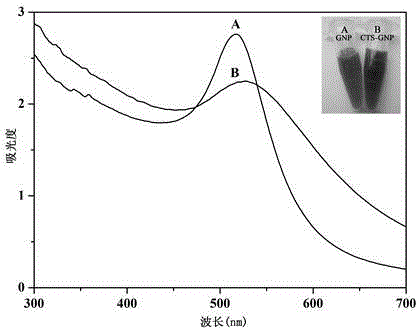
[0067] Example 2 Infrared Spectroscopy Detection to Characterize the Synthesized Gold Nanoparticle Composite
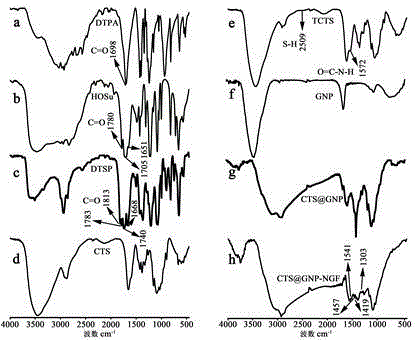
[0068] 1. Dry DTPA, HOSu, DTSP, CTS, TCTS, GNP, CTSGNP and CTSGNP-GNP, then put them into a mortar, add a certain amount of KBr, and grind the mixture evenly until the particle size is less than 2 μm, so as not to scatter light After that, put it into the dryer for drying treatment, press the mixture into a transparent sheet with a pressure of about 10MPa on the hydraulic press, and measure it on the machine.
[0069] 2. Results
[0070] The test results are attached figure 1 shown. figure 1 It is the infrared spectrogram of the corresponding reactants and products characterized by Fourier transform infrared spectrometer. Spectrum a is at 1698cm -1 There is a strong absorption peak, which is the C=O stretching vibration on the carboxyl group of 3,3'-dithiodipropionic acid; Spectrum b is at 1651 cm -1 、1705 cm -1 、1780 cm -1 The absorption peak is the stretchi...
Embodiment 3
[0071] Example 3 Characterization of the synthesized gold nanoparticle composite by Raman spectroscopy
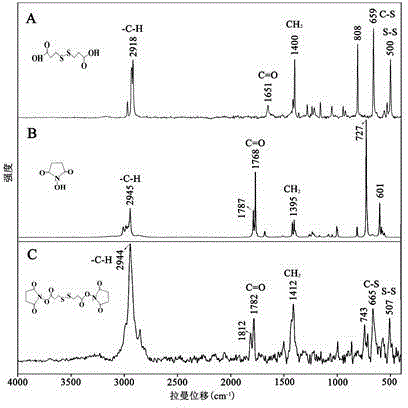
[0072] 1. Dry DTPA, HOSu and DTSP, and then take appropriate amount of samples on glass slides for detection on the machine.
[0073] 2. Results
[0074] The test results are attached figure 2 shown. figure 2 It is a Raman spectrum characterized by a Raman spectrometer to the reactant 3,3'-dithiodipropionic acid, N-hydroxysuccinimide and the product DTSP. Spectrum A at 659 cm -1 Raman peak at and 500 cm -1 The Raman peaks at are caused by the stretching vibrations of the C-S chain group and the S-S chain group in 3,3'-dithiodipropionic acid respectively, and there is no such Raman peak in spectrum B; while spectrum C is in 665cm -1 and 507 cm -1 The Raman peak at is caused by the stretching vibration of the C-S chain group and the S-S chain group in DTSP, which can prove the role of 3,3'-dithiodipropionic acid and N-hydroxysuccinimide in the dehydrating agent DCC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com