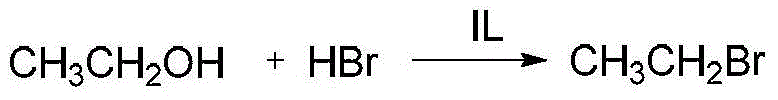Application of ionic liquid in bromoethane preparation
A technology of ionic liquid and ethyl bromide, applied in the field of green chemistry, can solve the problems of backward technology, environmental pollution, by-product dilute sulfuric acid, etc., and achieve the effect of simple post-processing, environmental friendliness and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-13
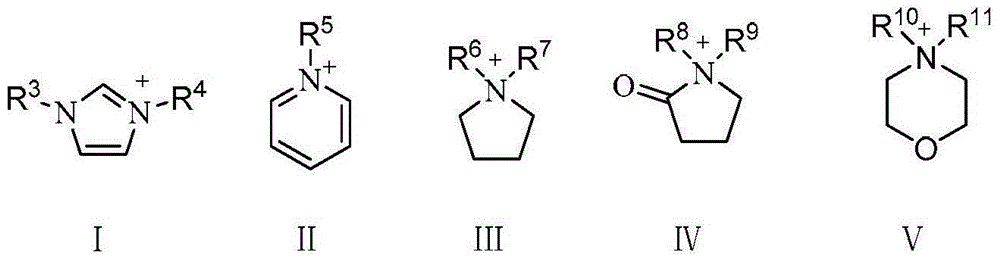
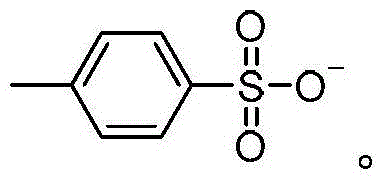
[0049] The synthesis steps of ionic liquid IL1-IL13 are as follows: N-methylimidazole, pyridine, N-methylmorphine, N-methylpyrrole and other cyclic tertiary amine compounds (referred to as cyclic tertiary amine) and excess chlorine N-butane was refluxed in toluene for 48 hours, and the obtained chloride salt was mixed with KBF respectively 4 、KPF 6 、CH 3 COOK, KHSO 4 , Potassium p-toluenesulfonate (p-TSAK) and other potassium salts are subjected to ion exchange reactions, and the obtained products are filtered and desolubilized, CH 2 Cl 2 The ionic liquids IL1-IL13 were obtained by extraction, vacuum drying, etc., and the reaction conditions and results are shown in Table 1.
Embodiment 14-15
[0051]The synthesis steps of ionic liquid IL14-IL15 are as follows: N-methyl-2-pyrrolidone, concentrated sulfuric acid, p-toluenesulfonic acid, etc. were heated to 50°C for 2 hours at a molar ratio of 1:1, and the product was washed with ethyl acetate for 3 hours after cooling. After vacuum drying, the products N-methylpyrrolidone bisulfate IL14 and N-methylpyrrolidone p-toluenesulfonate IL15 were obtained.
[0052] The reaction conditions and the result of table 1 embodiment 1~13
[0053]
[0054] The structures of the obtained ionic liquids IL1-IL15 are shown in the following formula:
[0055]
Embodiment 16-33
[0057] Embodiment 16-33 has investigated under different catalyst action, and ethanol and hydrobromic acid reaction prepare bromoethane, and its steps are as follows: in 500ml flask, add the hydrobromic acid of 270g60% and 40g protonic acid catalyst or ionic liquid, maintain material temperature At about 30°C, while stirring, add 120g of 95% ethanol dropwise to the system. Seal the reaction flask and place it in a water bath for magnetic stirring reaction. The temperature continues to rise to a boiling state. After the reaction is kept for 1 hour, the reaction flask is connected to a rectification device to separate bromoethane from the system, and the temperature at the top of the tower is controlled at 45 ~ Crude ethyl bromide began to collect at 46°C. When the temperature at the top of the tower rose to 60°C, the collection was stopped. The crude ethyl bromide obtained was washed twice with 10% NaOH solution, and rectified again to obtain the finished ethyl bromide. The r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com