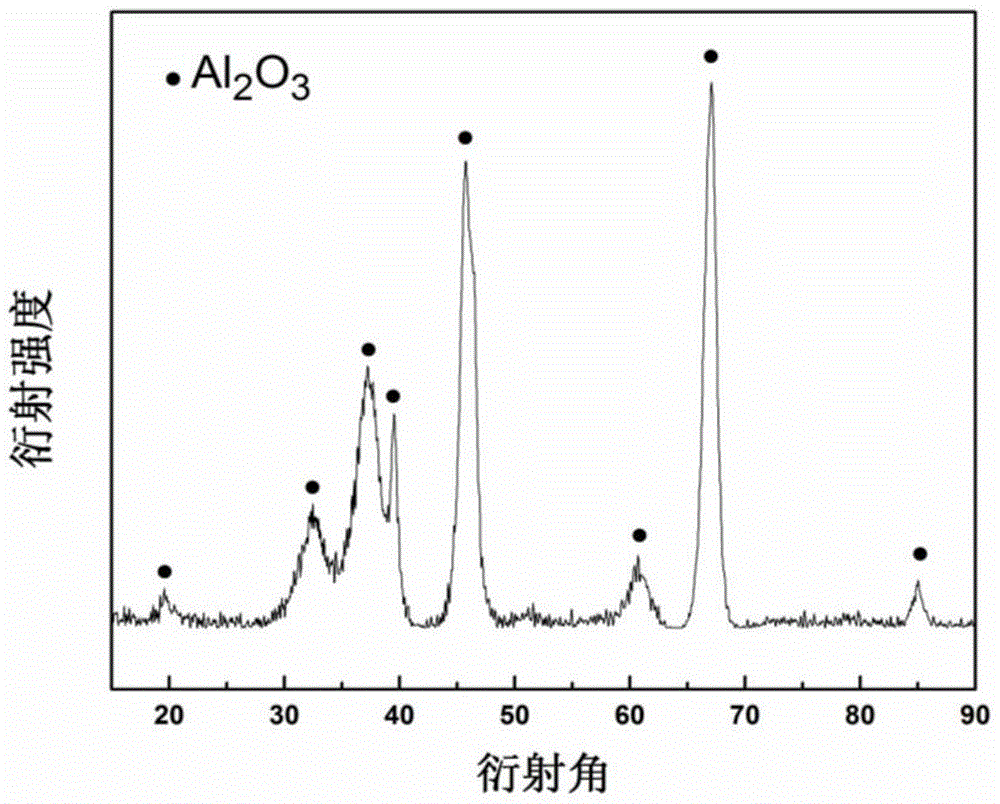
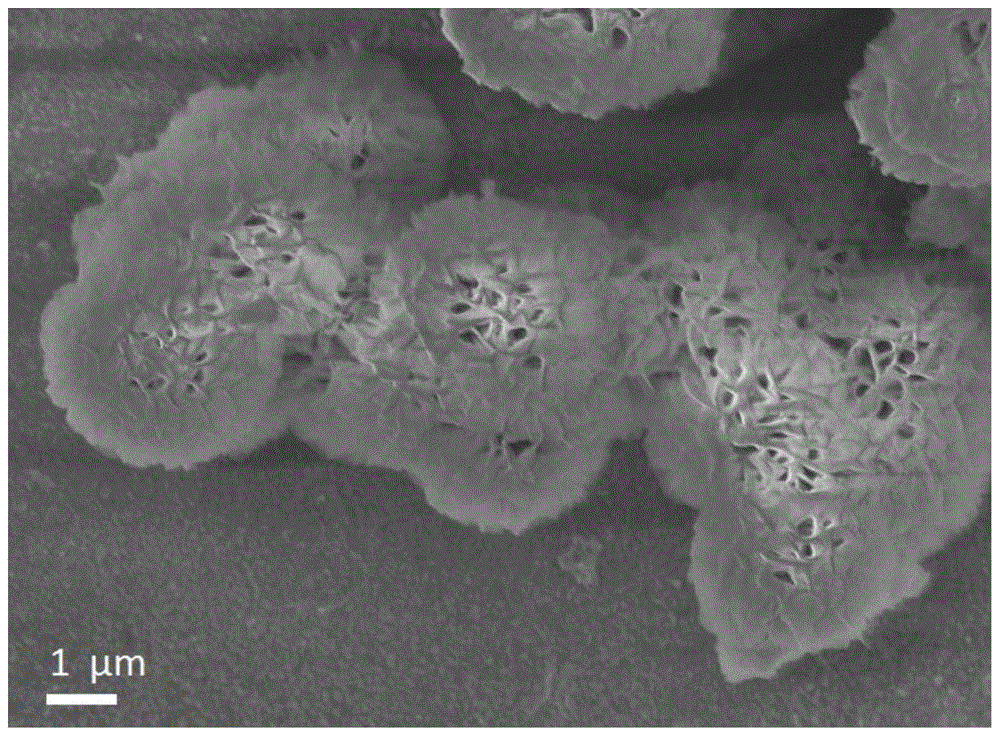
Preparation method of γ-alumina nanomaterial and nickel/γ-alumina catalyst
A kind of aluminum oxide nano-technology, applied in the direction of alumina/aluminum hydroxide, etc., can solve the problems of wide distribution of nano-alumina particle size, reduced catalytic activity of nano-alumina, and staying in the laboratory stage of nano-alumina. Achieve the effects of no pollution in the synthesis process, excellent anti-carbon deposition performance, and maintain anti-carbon deposition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of γ-alumina nanomaterials
[0040] At room temperature, add 0.8000g of alum and 0.4000g of urea to 25ml of deionized water, add a magnetic stirrer and stir for 10 minutes to completely dissolve the solid; transfer the solution to a 50ml reaction kettle, and perform a hydrothermal reaction at 180°C. The reaction time was 15 hours. After the hydrothermal reaction was completed and cooled down to room temperature, the reacted product was taken out, the precipitate was centrifuged, washed alternately with absolute ethanol and deionized water, and vacuum-dried at 60°C to obtain an AlOOH precursor. The AlOOH precursor is placed in a corundum porcelain boat and calcined in air to 800°C for 5 hours to obtain γ-alumina nanomaterials.
[0041] (2) Preparation of nickel / γ-alumina catalyst
[0042] 1.075g of prepared γ-alumina nanomaterials were impregnated in 100ml of 0.1M Ni(NO 3 ) 2 ·6H 2 O solution, stirred magnetically at room temperature until the solvent ...
Embodiment 2
[0052] (1) Synthesis of γ-alumina nanomaterials
[0053] At room temperature, add 0.8000g of alum and 0.1012g of urea into 25ml of deionized water, add a magnetic stirrer and stir for 10 minutes to completely dissolve the solid; transfer the solution to a 50ml reaction kettle, and perform a hydrothermal reaction at 180°C. The reaction time was 15 hours. After the hydrothermal reaction was completed and cooled down to room temperature, the reacted product was taken out, the precipitate was centrifuged, washed alternately with absolute ethanol and deionized water, and vacuum-dried at 60°C to obtain an AlOOH precursor. The AlOOH precursor is placed in a corundum porcelain boat and calcined in air to 800°C for 5 hours to obtain γ-alumina nanomaterials.
[0054] (2) Preparation of nickel / γ-alumina catalyst
[0055] 0.510g of prepared γ-alumina nanomaterials were impregnated in 100ml of 0.1M Ni(NO 3 ) 2 ·6H 2 O solution, stirred magnetically at room temperature until the solven...
Embodiment 3
[0057] (1) Synthesis of γ-alumina nanomaterials
[0058] At room temperature, add 0.8000g of alum and 0.8101g of urea to 25ml of deionized water, add a magnetic stirrer and stir for 10 minutes to completely dissolve the solid; transfer the solution to a 50ml reaction kettle, and perform a hydrothermal reaction at 180°C. The reaction time was 15 hours. After the hydrothermal reaction was completed and cooled down to room temperature, the reacted product was taken out, the precipitate was centrifuged, washed alternately with absolute ethanol and deionized water, and vacuum-dried at 60°C to obtain an AlOOH precursor. The AlOOH precursor is placed in a corundum porcelain boat and calcined in air to 800°C for 5 hours to obtain γ-alumina nanomaterials.
[0059] (2) Preparation of nickel / γ-alumina catalyst
[0060] 5.098g of prepared γ-alumina nanomaterials were impregnated in 100ml of 0.1M Ni(NO 3 ) 2 ·6H 2 O solution, stirred magnetically at room temperature until the solvent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com