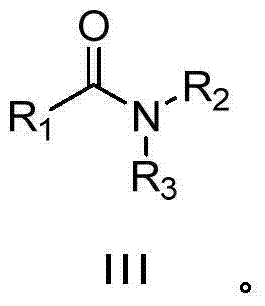
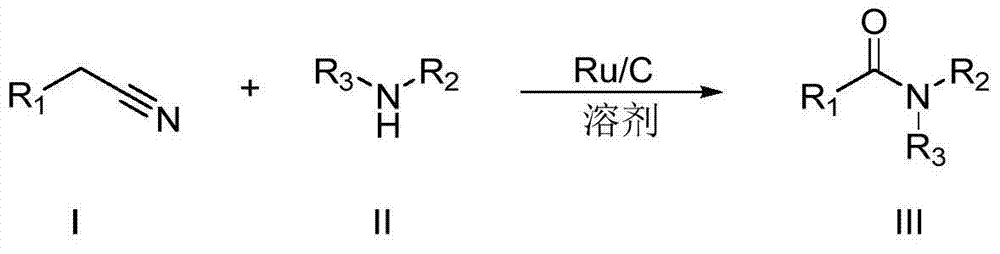
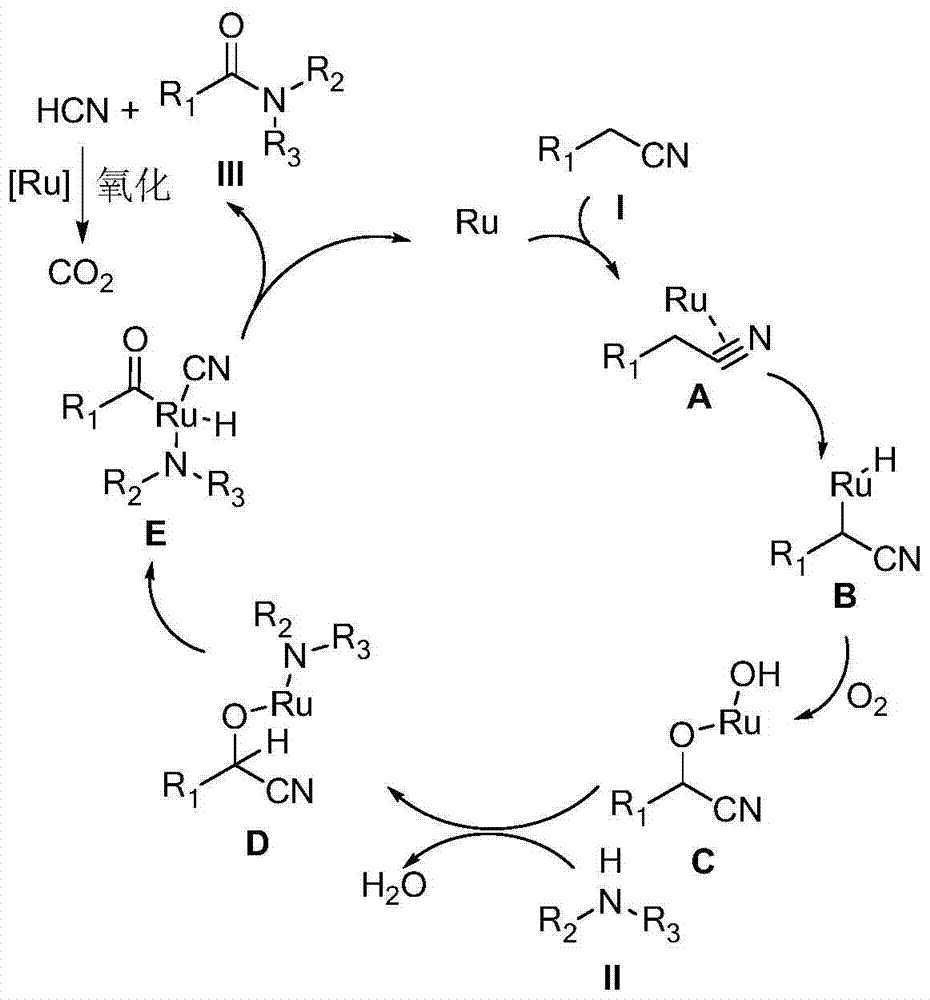
Synthesizing method for amide compound
A technology of amide compounds and synthesis methods, applied in the preparation of organic compounds, formation/introduction of amide groups, chemical instruments and methods, etc., can solve problems such as difficult separation, corrosion, and large amount of condensing agent, and achieve mild reaction conditions , raw materials are cheap and easy to obtain, and the effect of post-processing is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of N-n-butyl-benzamide (III-1)
[0031] The reaction formula is as follows:
[0032]
[0033] Under air atmosphere, add 0.005g (2.5‰mmol Ru) Ru / C catalyst to the reaction flask, then add 98μL (1mmol) n-butylamine (II-1), 10mL ethanol, and then mix 117μL (1mmol ) phenylacetonitrile (I-1) was added into the reaction flask, the temperature was kept at about 20° C., the reaction was stirred for 36 hours, and the reaction was monitored, and the raw material (I-1) basically reacted completely. After the reaction is finished, filter, wash the filter residue twice with ethanol, combine the filtrate, dry, concentrate, thin-layer chromatography (ethyl acetate / petroleum ether=1 / 5), obtain the N-normal compound shown in formula (III-1). Butyl-benzamide 0.149g, yellow solid, yield 84%, purity 98%. The structural representation of compound formula (III-1) is as follows:
[0034] 1 H NMR (600MHz, CDCl 3 )δ7.77-7.75(t,2H),7.49-7.46(t,1H),7.49-7.40(...
Embodiment 2
[0035] Embodiment 2: the preparation of N-n-butyl-benzamide (III-1)
[0036] Under air atmosphere, add 0.01g (5‰mmol Ru) Ru / C catalyst to the reaction flask, then add 98μL (1mmol) n-butylamine (II-1), 10mL ethanol, and then mix 117μL (1mmol ) phenylacetonitrile (I-1) was added in the reaction flask, the temperature was kept at about 20°C, and the reaction was stirred for 24 hours, and the reaction was monitored, and the raw material (I-1) basically reacted completely. Filter after the reaction finishes, wash the filter residue with ethanol twice, combine the filtrate, dry the organic phase, concentrate, thin-layer chromatography (ethyl acetate / petroleum ether=1 / 5), obtain the N shown in formula (III-1). -n-butyl-benzamide 0.152g, yield 86%, purity 98%.
Embodiment 3
[0037] Embodiment 3: the preparation of N-n-butyl-benzamide (III-1)
[0038] Under air atmosphere, add 0.005g (2.5‰mmol Ru) Ru / C catalyst to the reaction flask, then add 98μL (1mmol) n-butylamine (II-1), 10mL THF, and then mix 117μL (1mmol ) phenylacetonitrile (I-1) was added into the reaction flask, the temperature was kept at about 20° C., the reaction was stirred for 25 hours, and the reaction was monitored, and the raw material (I-1) basically reacted completely. Filter after the reaction is over, wash the filter residue twice with dichloromethane, combine the filtrates, extract twice with dichloromethane / water (30mL / 30mL) system, dry the organic phase, concentrate, thin-layer chromatography (ethyl acetate / petroleum ether= 1 / 5) to obtain 0.156 g of N-n-butyl-benzamide represented by formula (III-1), with a yield of 88% and a purity of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com