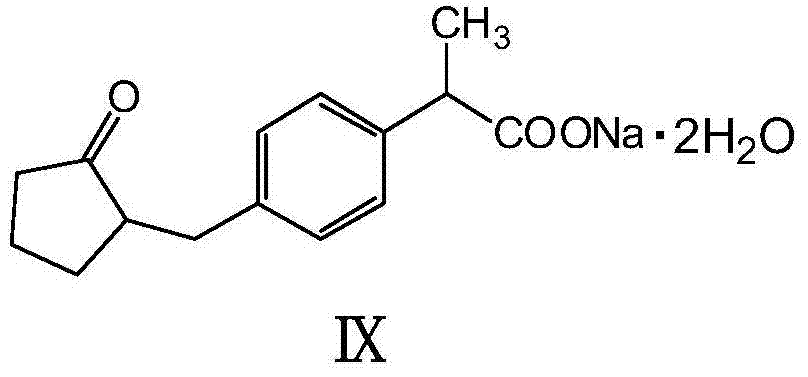
Synthetic methods of loxoprofen sodium and intermediate thereof
A technology of loxoprofen sodium and its synthesis method, which is applied in the field of medicine and chemical industry, and can solve the problems of affecting the quality of the final product, causing great environmental pollution, and having no industrial application value, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1 Preparation of Propiophenone (Compound I)
[0100] Add benzene (86ml) and ferric chloride (9.4g, 58.0mmol) successively in a 100ml three-necked flask, cool down in an ice bath, add propionyl chloride (8.9g, 96.0mmol) dropwise, and after feeding is complete, the oil bath is heated to 50 ~60°C, react for 10 hours. After the reaction was completed, the reaction solution was added into crushed ice, and then the pH of the solution was adjusted to 2-3. The above mixed solution was extracted 2-3 times with ethyl acetate, the organic layer was washed with water and saturated brine successively, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 12.3 g of propiophenone with a yield of 96%.
Embodiment 2
[0101] Embodiment 2 Preparation of Propiophenone (Compound I)
[0102] Add benzene (86ml) and aluminum trichloride (7.7g, 58.0mmol) successively in a 100ml three-necked flask, drop the temperature in an ice bath, add propionyl chloride (8.9g, 96.0mmol) dropwise, after feeding is complete, the oil bath is heated to 50 ~60°C, react for 10 hours. After the reaction was completed, the reaction solution was added into crushed ice, and then the pH of the solution was adjusted to 2-3. The above mixed solution was extracted 2-3 times with ethyl acetate, the organic layer was washed with water and saturated brine successively, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 11.9 g of propiophenone, with a yield of 93%.
Embodiment 3
[0103] Embodiment 3 Preparation of Propiophenone (Compound I)
[0104] Add benzene (86ml) and tin tetrachloride (15.1g, 58.0mmol) successively in a 100ml three-necked flask, cool down in an ice bath, add propionyl chloride (8.9g, 96.0mmol) dropwise, and after feeding is complete, the oil bath is heated to 50 ~60°C, react for 10 hours. After the reaction was completed, the reaction solution was added into crushed ice, and then the pH of the solution was adjusted to 2-3. The above mixed solution was extracted 2-3 times with ethyl acetate, the organic layer was washed with water and saturated brine successively, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 11.5 g of propiophenone with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com