Application of Fuzhuan tea in regulation of animal intestinal flora
A technology of intestinal flora and fucha, which can be applied to medical preparations containing active ingredients, plant/algae/fungus/moss ingredients, drug combinations, etc. Effects without toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of fu tea
[0034] 1. Preparation of spore suspension of S. coronis
[0035] Use a sterile inoculation needle to pick out the yellow obturator shells on Fuzhuan brick tea cubes and inoculate them on Chapei's medium, and cultivate them at 28°C. When cultured for 72 hours, pick a small amount of yellow closed cysts and separate them by streaking on a plate. After 2 to 3 streaks on a plate, the bacteria has been preliminarily purified. When the initially purified bacterial colony is in the vigorous growth period, use the inoculation loop to scrape the yellow occiput shell into a sterile vial filled with 10ml of sterile water, shake for 20min to make a uniform spore suspension and increase it by 10 times diluted to make 10 -3 ~10 -5 Diluted spore suspension.
[0036]Use a sterile pipette to draw 0.1ml of the spore suspension of each dilution and spread it on the blank Chapeau medium, and then culture it at 28°C. Select a plate with only 1 ...
Embodiment 2
[0044] Example 2: Function experiment of Fu tea regulating human intestinal flora
[0045] According to the Ministry of Health "Technical Specifications for Testing and Evaluation of Health Drinks (2003)", the method for evaluating the function of intestinal flora in human body is tested and evaluated for the Fucha prepared in Example 1, as follows:
[0046] 1. Drinking experiment group
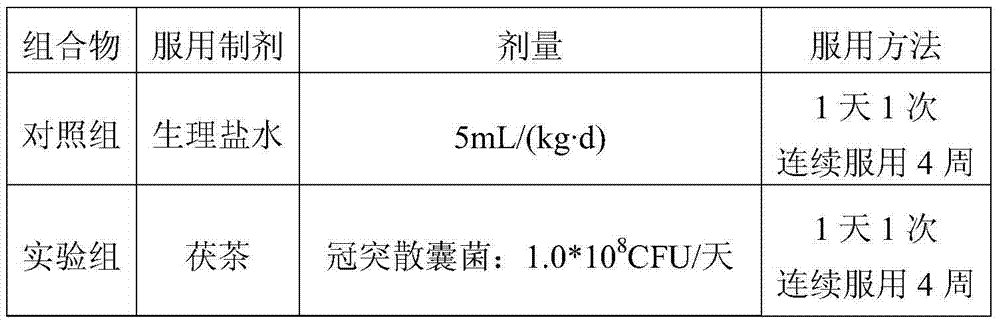
[0047] Table 1 Grouping table of drinking experiment
[0048]
[0049] 2. Human body drinking test method
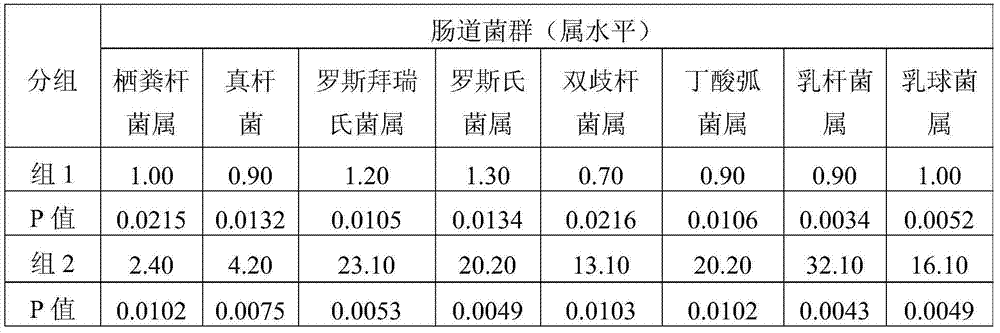
[0050] The subjects were randomly divided into two groups, 20 people in each group. Before the subjects tried to drink, the feces of the subjects were collected aseptically, and the intestinal flora was tested by 16S rDNA sequencing as a reference for the background level.
[0051] According to the grouping situation in Table 1, group 1 took the control group and group 2 took the experimental group. After 4 weeks, the feces of the subjects were collected, and 16SrDNA sequencing was...
Embodiment 3
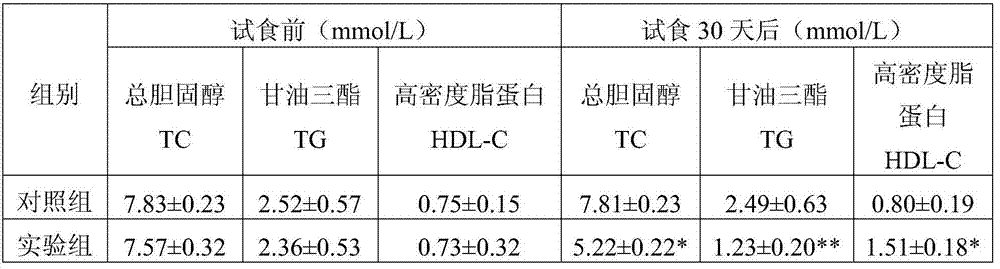
[0060] Example 3: Fu tea lowering blood fat human body food trial experiment
[0061] According to the Ministry of Health's "Health Food Inspection and Evaluation Technical Specifications (2003)", the method for evaluating the blood fat-lowering function human body test experiment evaluation method is used to evaluate the Fu tea prepared in Example 1, as follows:
[0062] 1. Human body test food and test method:
[0063] 20 cases of type II hyperlipidemia were selected on a voluntary basis, including 16 males and 4 females, aged from 43 to 75 years old, without serious complications such as heart, liver and kidney, and a comparative design was adopted before, during, and after eating.
[0064] The subjects were randomly divided into 2 groups, 10 people in each group. The types of therapeutic drugs, diet control and activities used by the subjects were unchanged. The experimental group took the Fu tea of the present invention, and the control group took normal saline. The dos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com