Synthesis method for lupeol
A technology of lupeol and its synthesis method, which is applied in the production of steroids, bulk chemicals, organic chemistry, etc., can solve the problems of difficult application of industrial production of lupeol, harsh reaction conditions, and low yield, and achieve high yield High, less experimental steps, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
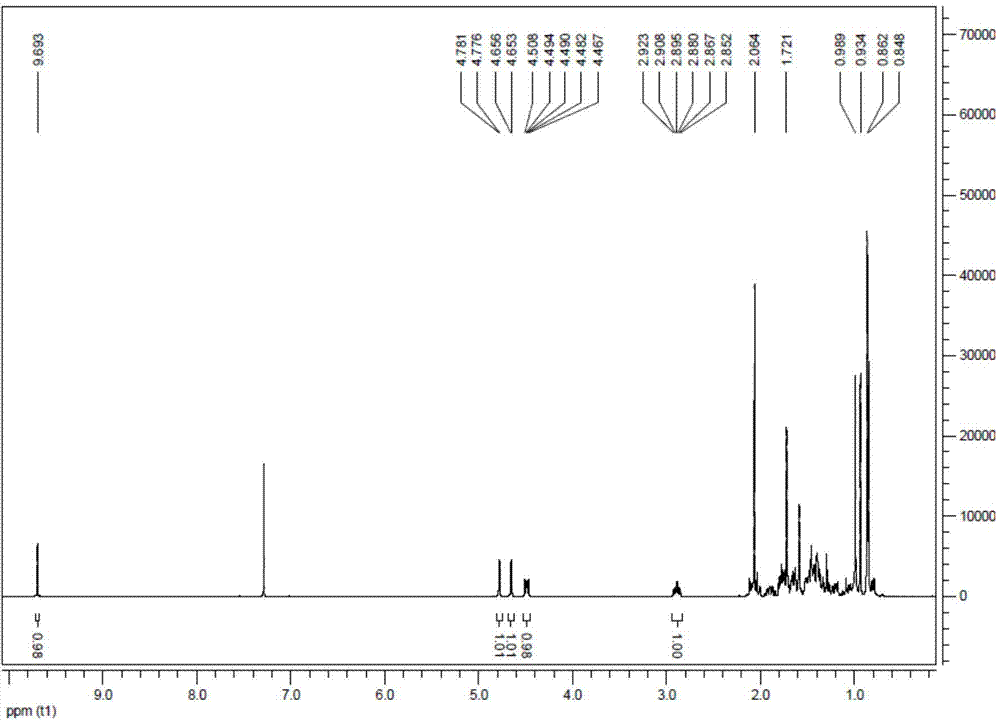
[0038] Place 40 mg of 3-O-acetyl betulin aldehyde in a single-necked bottle at room temperature, add 5 ml of diethylene glycol and heat to dissolve, add 30 ul of hydrazine hydrate, stir at 80°C for 3 hours. After TLC monitoring and other raw material spots completely disappeared, 32 mg of sodium hydroxide was added, and stirred at 200° C. for 5 h. TLC monitors that the reaction is complete, stop the reaction, add 20ml of ethyl acetate to the reaction solution to dissolve, wash with 40ml of saturated saline three times, combine the organic layer and concentrate under reduced pressure, and use petroleum ether: ethyl acetate = 15:1 as the eluent for silica gel By column chromatography, 20 mg of lupeol was obtained, with a yield of 20%. 1 H NMR (500MHz, CDCl 3 ): δ4.71(s,1H),4.58(s,1H),3.21(dd,1H,J=5H Z ),2.40(m,1H),1.89-1.98(m,1H),1.70(s,3H),1.05(s,3H),0.98(s,3H),0.97(s,3H),0.85(s,3H ),0.81(s,3H),0.78(s,3H). 13 C NMR (125MHz, CDCl 3 )δ150.97,109.34,79.01,55.29,50...
Embodiment 2
[0040]
[0041] Place 30 mg of 3-O-acetyl betulin aldehyde in a single-necked bottle at room temperature, add 5 ml of DMSO (dimethyl sulfoxide) and heat to dissolve, add 30 ul of hydrazine hydrate, stir at 80 ° C for 3 h. After TLC monitoring and other raw material spots completely disappeared, 23 mg of potassium tert-butoxide was added and stirred at 140° C. for 5 h. TLC (petroleum ether: ethyl acetate = 4:10) monitored the completion of the reaction, stopped the reaction, added 20ml ethyl acetate to the reaction liquid to dissolve, washed with 40ml saturated brine three times, combined the organic layer and concentrated under reduced pressure, and then mixed with petroleum ether: acetic acid Ethyl ester=15:1 was used as the eluent to perform silica gel column chromatography to obtain 10 mg of lupeyl alcohol with a yield of 34%. 1 H NMR (500MHz, CDCl 3 ): δ4.71(s,1H),4.58(s,1H),3.21(dd,1H,J=5H Z ),2.40(m,1H),1.89-1.98(m,1H),1.70(s,3H),1.05(s,3H),0.98(s,3H),0.97(s,3H),0.8...
Embodiment 3
[0043]
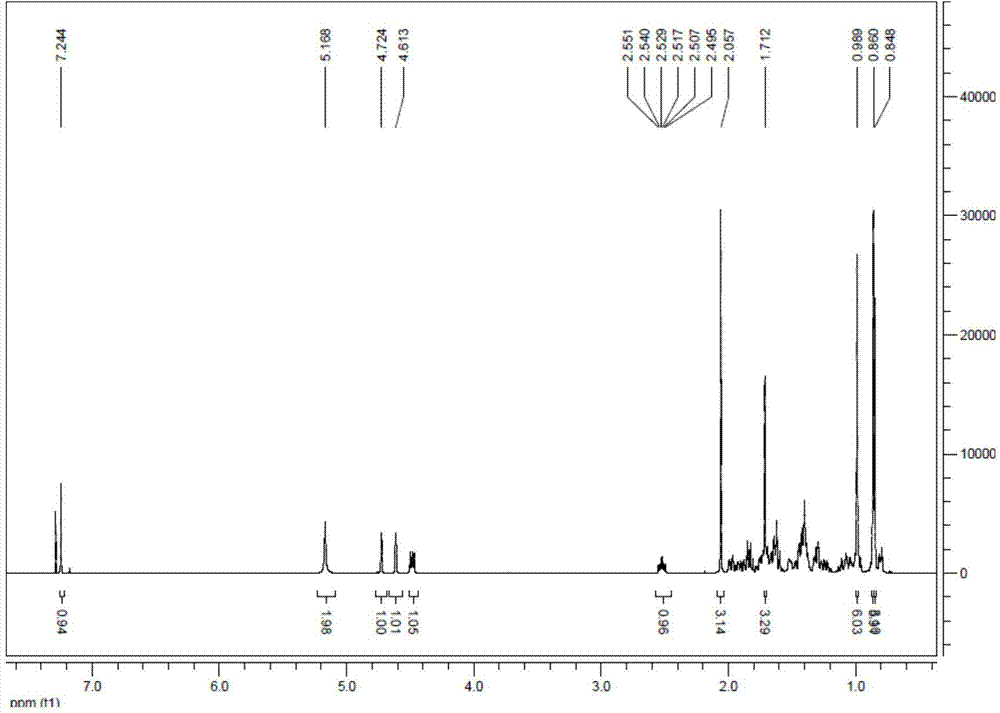
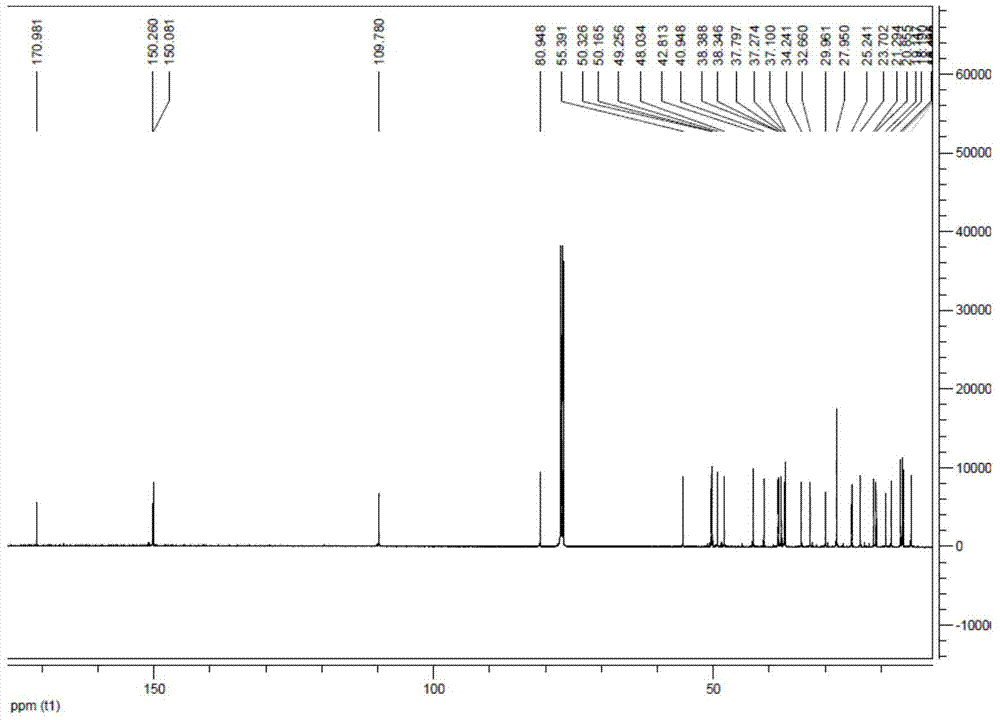
[0044] 100mg of 3-O-acetyl betulin was dissolved in 5ml of ethanol at room temperature, 200ul of hydrazine hydrate was added, heated and refluxed for 3h, the reaction was stopped and cooled to room temperature, 20ml of ice water was added to separate out the precipitation to obtain 100mg of intermediate hydrazone, (yield 98 %.1H NMR (500MHz, CDCl3): δ7.24(s,1H) 5.16(s,2H), 4.72(s,1H), 4.61(s,1H), 4.80(dd,1H, J=5HZ), 2.53(m, 1H), 2.06(s, 3H), 1.70(s, 3H), 0.98(s, 6H), 0.86(s, 6H), 0.865(s, 3H) 13C NMR(500MHz, CDCl3)δ170. 98,150.26,150.08,109.78,80.95,55.39,50.33,50.17,49.26,48.03,42.81,40.95,38.39,38.35,37.79,37.28,37.10,34.24,32.66,29.96,27.96,25.24,23.70,21.30,20.86,19.15, 18.20, 16.49, 16.15, 16.05, 14.63; MS(ESI)[M+H] + 497.5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com