Protein-based pharmacological active substance composition, and preparation method and applications thereof
A pharmacological activity, protein technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problem of not improving the biodistribution of paclitaxel molecules and blood circulation time, and achieve the enrichment effect and biodistribution improvement, inhibited growth, Strong anti-proliferative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The preparation method of the pharmacologically active substance preparation provided by the present invention for in vivo delivery comprises:
[0056] An aqueous medium in which a pharmacologically active substance, a water-miscible organic solvent, and a protein is mixed to obtain a mixture (without surfactants in the mixture) and subjected to pressures ranging from 3,000 to 30,000 psi 2 in the high pressure homogenizer. The organic solvent can be selectively removed from the mixture after being subjected to high shear conditions; it can also be selectively removed from the mixture before being subjected to high shear conditions.
[0057] The above-mentioned process of forming the mixture can be that the organic solution is added to the aqueous solution, and the aqueous solution can also be added to the organic solvent. The conditions in the mixing process can be ultrasonic, magnetic stirring, mechanical stirring, homogenization or high shear agitator, There could also...
preparation example 1
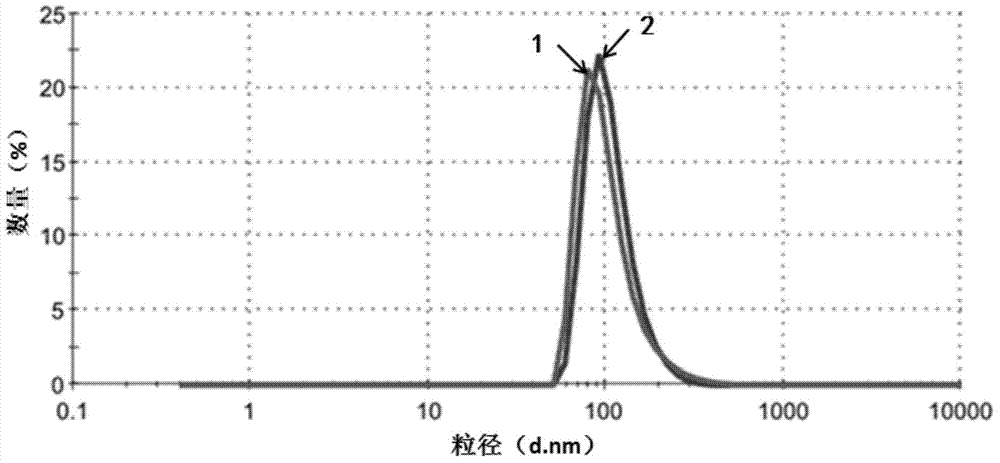
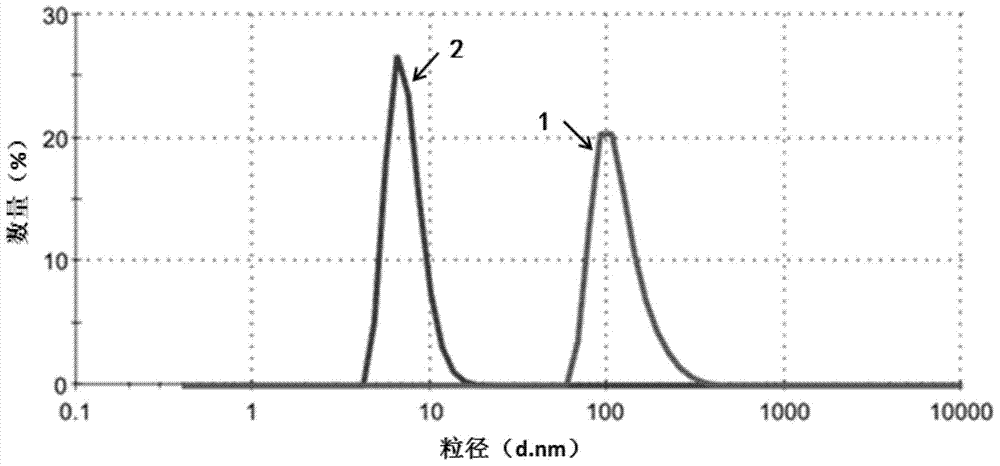
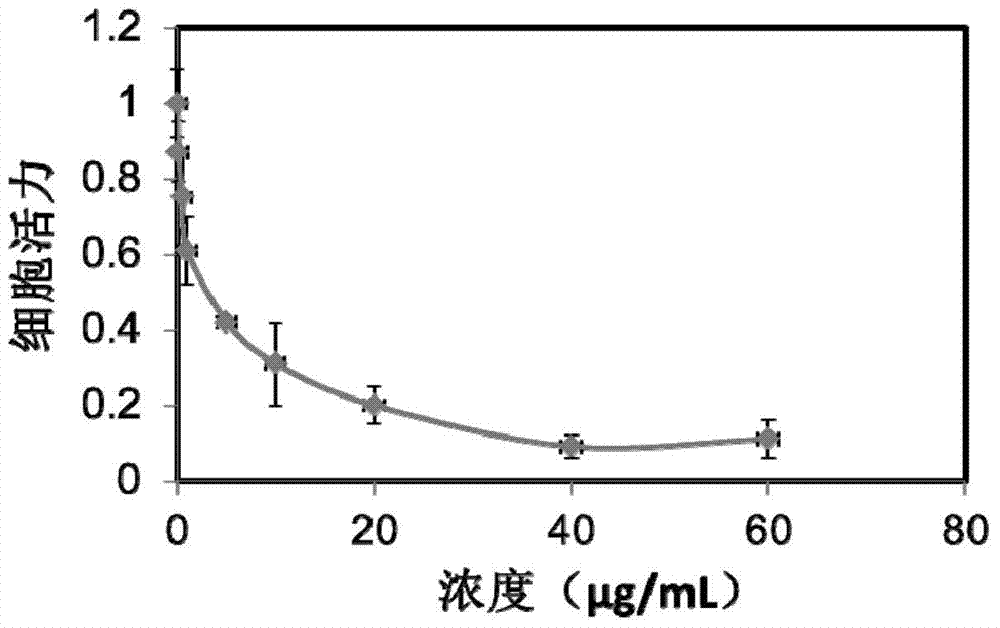
[0074] The purpose of this example is to demonstrate the homogeneous preparation of nanoparticles using high pressure. Dissolve 30 mg of paclitaxel (Paclitaxel) in 3.0 ml of ethanol. To the solution was added 27.0 ml of an aqueous solution of human serum albumin (1% w / v). The resulting mixture was placed in a Rotary evaporator and evaporated at 40°C under reduced pressure (30mmHG) for 20-30 minutes to remove ethanol. Homogenization was then performed at low RPM for 5 minutes to form a coarse emulsion, which was then transferred to a high pressure homogenizer (Avestin). Then at 9000-18000 psi 2 (psi) at least five more high-pressure homogenization cycles. The resulting dispersion was translucent, and the paclitaxel particles generally had a diameter of less than 200 nm (quantitative statistics, Malvern Zetasizer). Without adding any antifreeze, the dispersion was freeze-dried to form a dry powder. The dry powder can be reconstituted with sterile water or physiological sali...
preparation example 2
[0076]The purpose of this example is to demonstrate the use of cavitation and high shear stress in sonication for the preparation of paclitaxel nanoparticles. Dissolve 20 mg paclitaxel in 2.0 ml ethanol. Add 4.0 ml of human serum albumin solution (5% w / v) to the solution. The mixture was homogenized at low RPM for 5 minutes to form a coarse emulsion, which was then transferred to a 40 kHz sonication cell. Sonicate for 1 min at 60-90% power, level 0. The mixture was then transferred to a rotary evaporator and evaporated at 40°C under reduced pressure (30 mmHg) for 20-30 minutes to remove the organic solvent. Paclitaxel microparticles were obtained, typically less than 200 nm in diameter (quantitative statistics, Malvern Zetasizer). Without adding any antifreeze, the dispersion was freeze-dried to form a dry powder. The dry powder can be reconstituted with sterile water or physiological saline, and the particle size in the reconstituted solution remains below 200nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com