Composition comprising rebamipide as active ingredient for preventing or treating hyperlipemia and diseases associated therewith
A technology for hyperlipidemia and rebamipide, applied in the field of rebamipide, can solve problems such as side effects, and achieve the effects of improving fatty liver, increasing expression, and improving blood lipids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
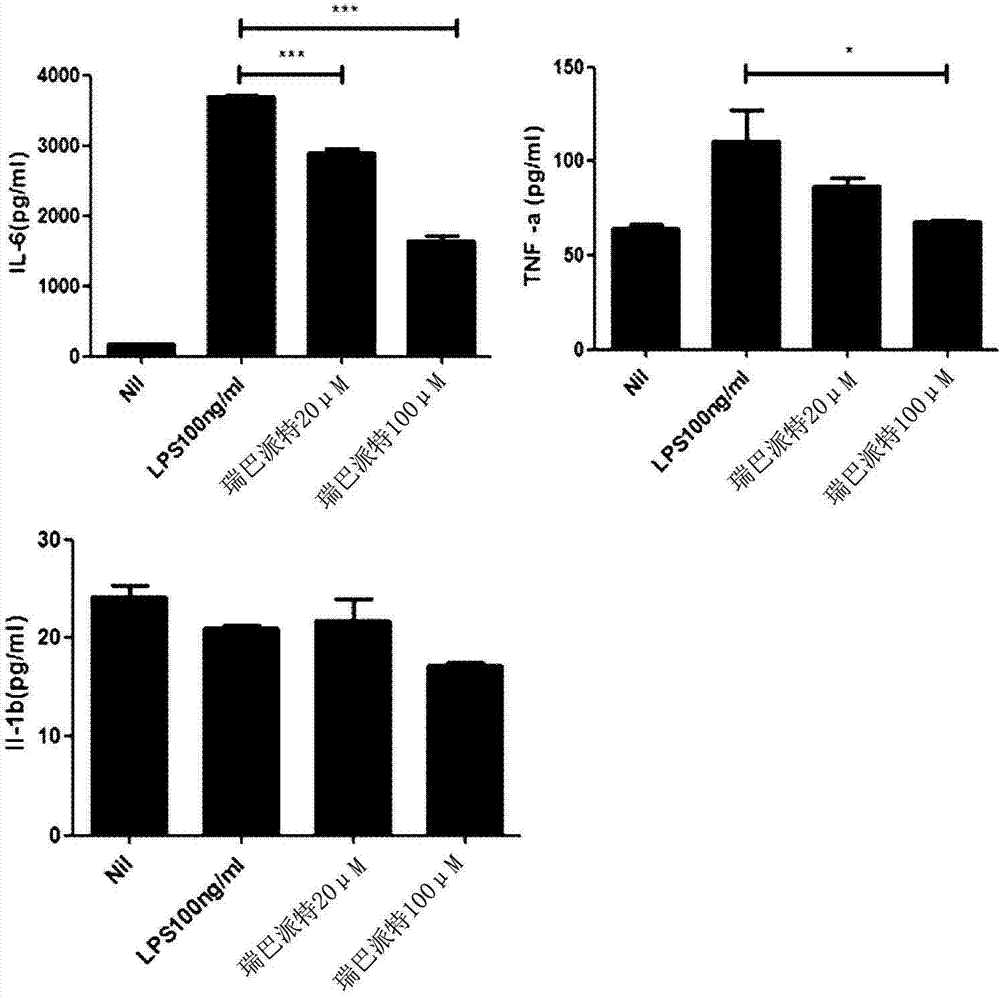
[0096] Inflammatory cytokines based on rebamipide treatment of macrophages (inflammatory cytokines) generation inhibitory effect
[0097] In order to ascertain the effect of rebamipide on macrophages, the inventors of the present application pre-treated mouse (Mouse) macrophages with rebamipide, and utilized LPS (lipopolysaccharide; lipopolysaccharide) to stimulate, Thus, the inflammatory cytokines TNF-a (tumor necrosis factor-α; tumor necrosis factor-alpha), IL-6 (interleukin-6; interleukin-6) and IL-1β (interleukin-1β; The degree of production of interleukin-1β) was assessed by ELISA (enzyme linked immunosorbent assay) analysis.
[0098] Cell culture
[0099] RAW 264.7 cells, which are mouse macrophage cell lines, were distributed from the Korean Cell Line Bank (KCLB), and for cell culture, a culture medium containing 10% FBS and 1% penicillin-streptomycin (penicillin-streptomycin) was used. DMEM (Dulbeccos Modified Eagle Medium) medium. At 37°C, 5% CO 2 cells were...
Embodiment 2
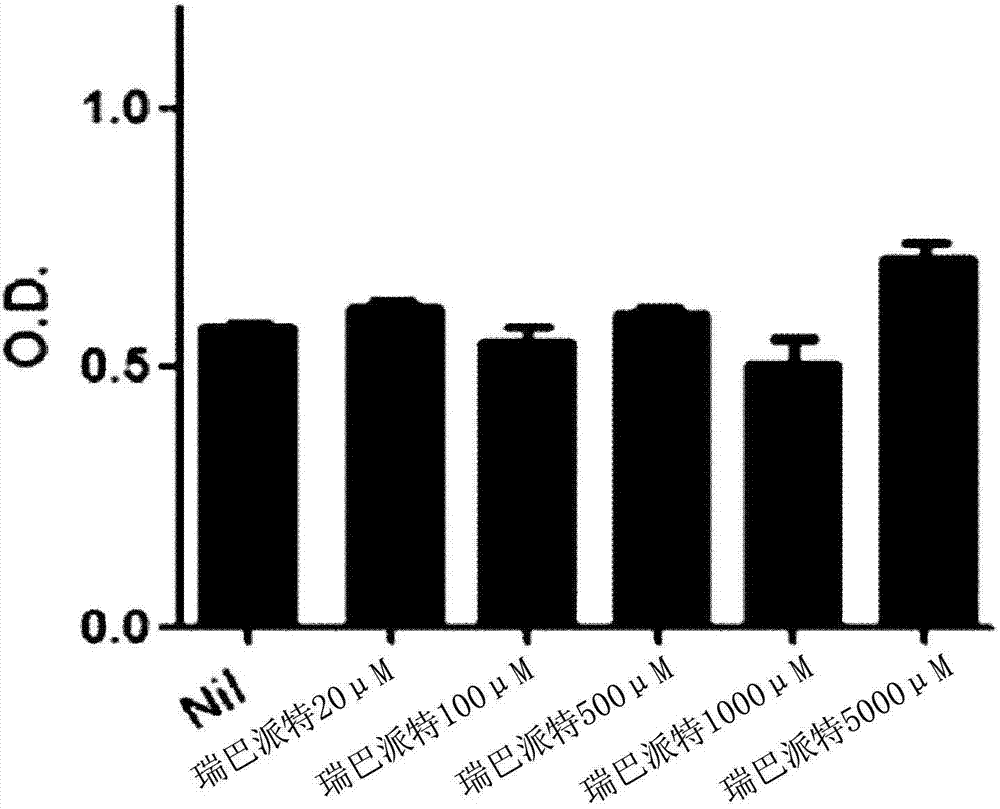
[0106] Toxicity determination of rebamipide to cells
[0107] In order to examine the cytotoxicity of rebamipide against RAW 264.7, which is a mouse macrophage, an MTT assay (MTT assay) was performed.
[0108] This method measures the conversion of MTT (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide; thiazolium blue) to formazan (Formazan) in a 96-well Packed in the plate 1×10 4 The cells / well (cells / well) of RAW 264.7 cells were treated with rebamipide according to the concentration (20, 100, 500, 1000, 5000 μM) for 18 hours. Add 100 μl of MTT solution to each well (well), so that at 37 ° C, 5% CO 2 The reaction was carried out in an incubator for 4 hours, and then the change in absorbance was measured at 570 nm using a microplate reader (VERSAmax, Molecular Devices, USA).
[0109] As a result, such as figure 2 As shown, the cytotoxicity is weak at concentrations of 20 μM, 100 μM and above, which are effective concentrations, and thus it can be seen that th...
Embodiment 3
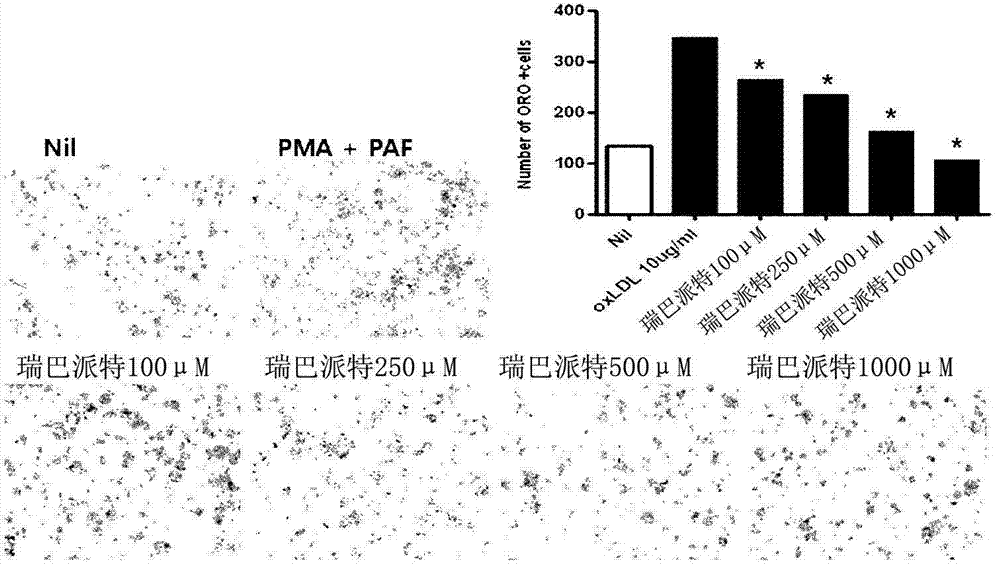
[0111] Inhibitory effect of rebamipide-based treatment on vascular etiological cells
[0112] In this experiment, it was examined whether the generation of vascular etiogenic cells could be really inhibited when rebamipide treatment was performed in the vascular etiogenic cells. The "vascular etiological cell" refers to a foam cell (Foam cell) that forms a precursor cell of an arteriosclerotic plaque that causes arteriosclerosis, and is considered to be a cell that causes arteriosclerosis. In the invention, it is arbitrarily named as vascular etiological cell.
[0113] Inhibitory effect of rebamipide-based treatment on vascular etiological cells
[0114] In order to carry out this experiment, THP1 cells, a human macrophage cell line, were treated with PMA (phorbol 12'-myristate 13'-acetate; 12'-myristate-13'-acetate phorbol) at a concentration of 160nM to activate the cells , and then PAF (platelet-activating factor; platelet-activating factor) was treated with 10ug / ml,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com