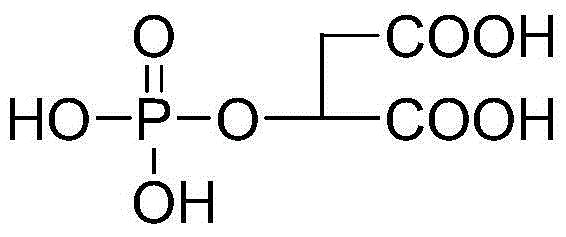
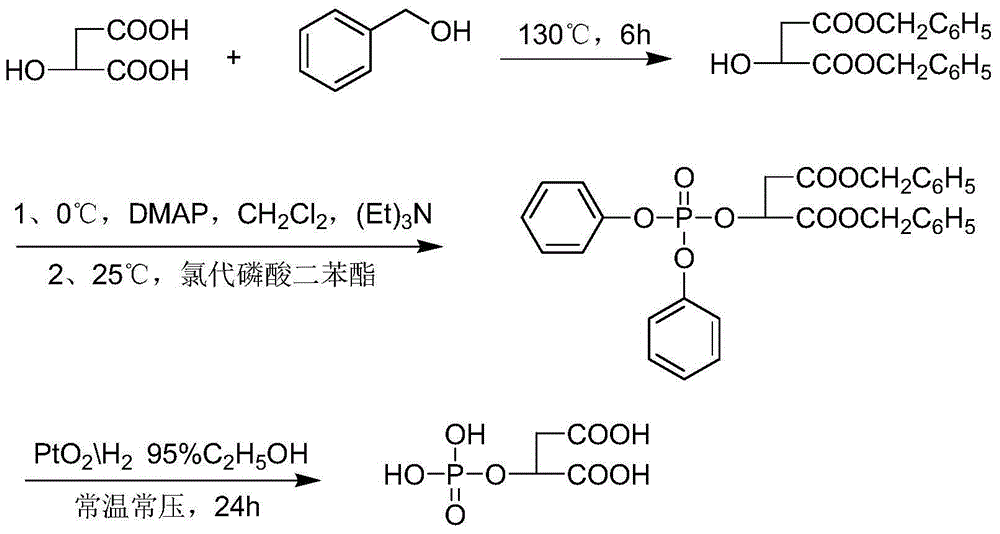
Malic acid phosphate ester and applications of malic acid phosphate ester in inhibition of calcium ion deposition diseases
A technology of phosphate malate and dibenzyl malate, which is applied in the field of chemical biology, can solve the problems of poor fat solubility and low stability, and achieve the effect of reducing the concentration of calcium ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1. Preparation of malic acid phosphate.
[0018] Weigh 1g of D-L malic acid, add 0.01g of p-toluenesulfonic acid under the protection of nitrogen, put it into an oil bath and stir. Raise the temperature to about 130°C until the solid becomes molten. Continue to stir for about 10 minutes. After the malic acid is completely liquid, put it into an oil bath at 80°C. 1.6 g of benzyl alcohol was added. The temperature was slowly raised to 120°C, and the reaction progress was tracked by thin-layer chromatography, and the reaction was terminated after 6 hours. After cooling to room temperature, a saturated sodium bicarbonate solution was slowly added dropwise to the reaction liquid until neutral. Add anhydrous diethyl ether for extraction until the white substance in the solution disappears completely. The extract was washed three times with saturated brine, and dried over anhydrous magnesium sulfate overnight. Add 8 mL of re-distilled dichloromethane to dissolve,...
Embodiment 2
[0019] Example 2. Inhibitory effect of malate phosphate on C2C12 calcification in mouse myoblasts.
[0020] Establish cell model, its method is as follows: First, mouse myoblast C 2 C 12 Take 5×10 5 cells / ml were inoculated in 24-well plates, and the next day, serum-free medium was added and cultured in an incubator for 24 hours. On the third day, use 1mCi / ml45Ca 2+ The labeled medium was exchanged for cells, and then ATP was added at a final concentration of 1 mM. Negative controls were performed with cells not treated with ATP or β-glycerophosphate. After 48 or 72 hours, the medium was discarded, the cells were washed 5 times with low-temperature Hank's equilibrium solution, and 0.1N NaOH was added. Finally, the amount of radioactive substance in the cell lysate was determined, and the experiment was carried out three times, and the average value was calculated. The results are shown in Table 1.
[0021] Table 1: Malate phosphate inhibits C 2 C 12 Study on Cell Cal...
Embodiment 3
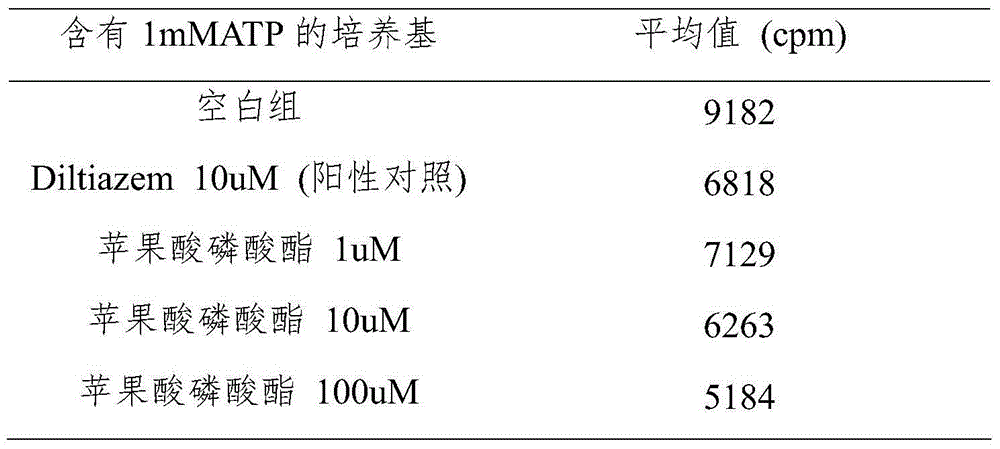
[0023] Example 3. Reducing effect of malate phosphate on calcium ion concentration in HSK of human skeletal muscle cells.
[0024] Human skeletal muscle cells HSK were seeded in a 96-well plate at 10,000 / well, cultured in a medium containing malate phosphate or calcium channel inhibitor Diltiazem for 48 hours, and then added Fluo-4 calcium ion fluorescent indicator, And placed in a fluorescence plate reader to measure the fluorescence intensity. The intracellular calcium ion concentration is directly proportional to the fluorescence intensity. Three experiments were carried out in this way, and the average value was calculated at the end. The results are shown in Table 2.
[0025] Table 2: Study on reduction of calcium ion concentration in HSK cells by malate phosphate
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com