Preparation method of coating catalyst and coating catalyst prepared by using method
A technology of coating catalysts and catalysts, applied in the direction of catalyst activation/preparation, preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of difficult to obtain mechanical strength catalysts, easy peeling of coatings, etc., achieve high strength, improve The effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Preparation of catalyst
[0049] Add 490.50g molybdenum trioxide (MoO 3 ), 23.44g vanadium pentoxide (V 2 o 5 ), the phosphoric acid (H of 41.08g 85wt% mass concentration 3 PO 4 ), 24.71g 80wt% arsenic acid (H 3 AsO 4 ), 0.88g boric acid (H 3 BO 3 ), stirred and refluxed at 98°C for 1h, then added 2.40g of copper phosphate (Cu 3 (PO 4 ) 2 ·3H 2 O), 0.69g cuprous oxide (Cu 2 0), continue to reflux and stir to obtain a blue-green clear solution after 10h, the solution is spray-dried on an airflow spray dryer, the atomizing airflow pressure (absolute pressure) is 0.35MPa, and the speed of compressed air ejection from the nozzle is 1500m / s, the inlet temperature of the spray dryer is 140-150°C, the outlet temperature is 70-80°C, and the average particle size of the obtained catalyst powder is 2.5μm.
[0050] Get 200g of catalyst powder and disperse in 100g of ethanol with a water content of 5wt% to obtain slurry A (viscosity 100mPa·s), get 40g of polyvinyl...
Embodiment 2
[0055] Add 490.50g molybdenum trioxide, 20.84g vanadium pentoxide, 0.66g tungsten trioxide, 43.57g 85% mass concentration phosphoric acid, 29.65g 80wt% arsenic acid to 4000g deionized water, stir and reflux at 98°C for 1h Add 3.60g of copper phosphate, 1.04g of cuprous oxide and 3.35g of cupric oxide, and continue to reflux and stir for 10 hours to obtain a blue-green solution. The solution is spray-dried on an air-flow spray dryer. The atomizing airflow pressure is 0.25MPa, compressed The speed of air ejected from the nozzle is 1000m / s, the inlet temperature of the spray dryer is 140-150°C, the outlet temperature is 70-80°C, and the average particle size of the obtained catalyst powder is 3.2μm.
[0056] Get 200g catalyst powder and disperse in 120g dehydrated alcohol to obtain slurry A (viscosity 90mPa s), get 35g polyvinylpyrrolidone (average molecular weight is about 100,000) and dissolve in 40g dehydrated alcohol to obtain solution B (viscosity 150mPa s ), slurry A and so...
Embodiment 3
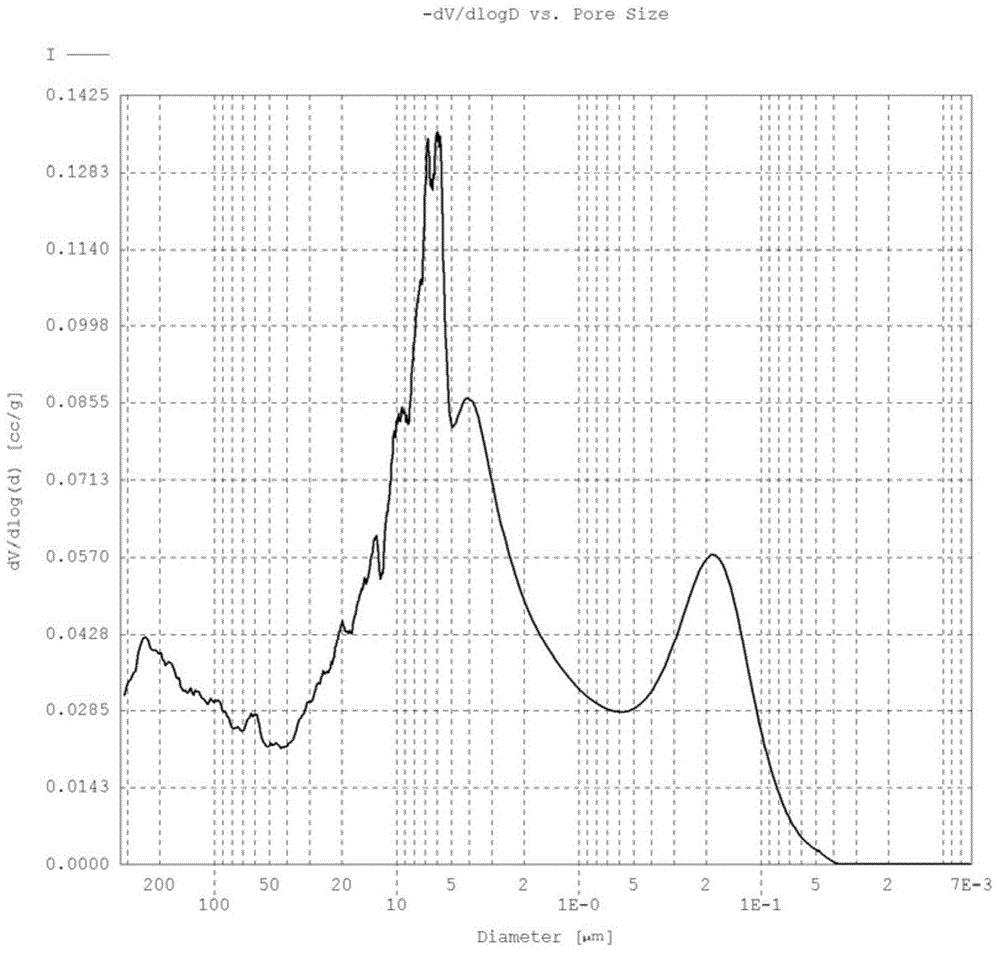
[0072] The catalyst was prepared with reference to Example 2, the difference being that the calcination procedure was as follows: stay in an air atmosphere at 50°C for 8h, stay in a nitrogen atmosphere at 350°C for 1h, and stay in an air atmosphere at 300°C for 3h. Finally, the composition of the heteropolyacid is obtained: Mo 12 P 1.4 V 0.8 Cu 0.3 As 0.6 W 0.01 o x . The wear index test result of the coated catalyst is 0.6%; the pore volume contained in the pores with a pore diameter of 100-2000 nm in the active component of the heteropoly acid outer layer accounts for 75% of the total pore volume, and the pore volume contained in the pores with a pore diameter of 100-500 nm The pore volume accounts for 35% of the total pore volume of the active components of the outer layer.
[0073] The methacrolein oxidation reaction was carried out in the same manner as in Example 1. After 50 hours of continuous reaction, the conversion rate of methacrolein was 80.1%, and the selec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com