Synthesis Method of Hydrophilic Ion Pair Chromatography Packing
A technology of ion-pair chromatography and synthesis method, applied in the field of ion-pair chromatography packing, can solve the problems of difficult control of sulfonation reaction, poor product consistency, low success rate, etc. high pressure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
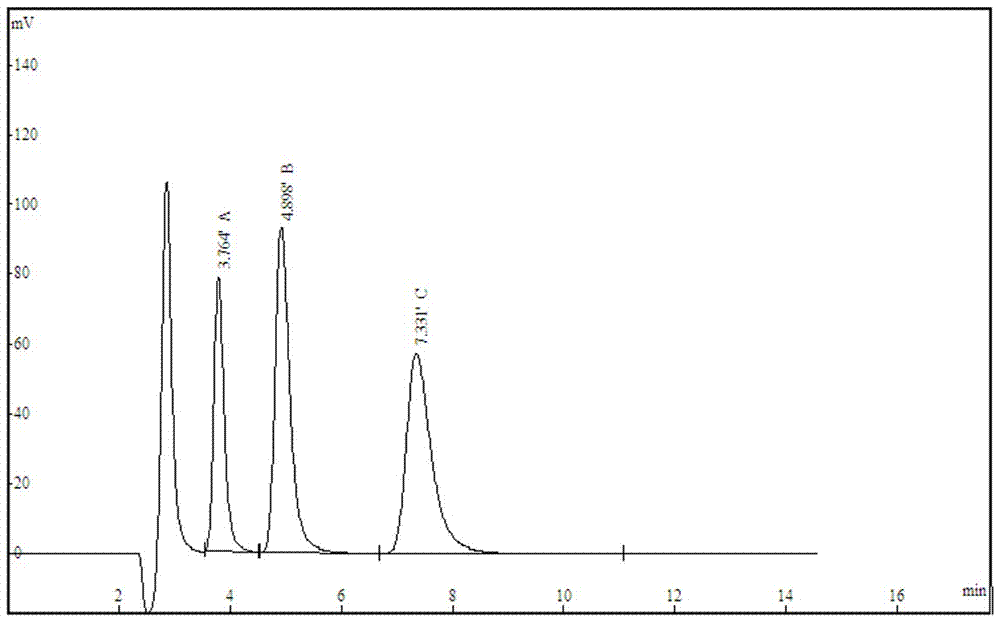
Image
Examples
Embodiment 1
[0021] Add 3 g of protective colloid reagent polyvinyl alcohol to 500 ml of deionized water, and stir to dissolve. Divinyl benzene with a content of 80% and initiator (di)benzoyl peroxide dissolved in toluene were packed into a brown bottle, filled with nitrogen gas and sealed, and stored at 4 degrees Celsius for later use.
[0022] In a 1000 ml three-neck flask equipped with a stirrer and a reflux condenser, add the dissolved polyvinyl alcohol solution in Part 1, then add 5 ml of the mixed monomer and seeds in Part 1, and first heat it at 30 degrees Celsius under a nitrogen atmosphere. Stir and swell for 24 hours, then stir and react at 80 degrees Celsius for 4 hours, and finally mature at 90 degrees Celsius and stir and react for 10 hours, take out, wash, filter and dry to obtain an ethylstyrene-divinylbenzene polymer with a crosslinking degree of 80%. Microspheres.
[0023] Get 10 grams after drying the ethylstyrene-divinylbenzene polymer microspheres prepared in 2, ...
Embodiment 2
[0029] The difference from Example 1 is that the protective colloid reagent is gelatin, 500 milliliters of deionized water is dissolved in 5 grams of protective colloid reagent gelatin, and the divinylbenzene with a content of 70% and the initiator are lauryl peroxide.
[0030] The difference between 2 and Example 1 is that the gelatin solution dissolved in 1 is added to the three-necked bottle; under nitrogen atmosphere, first stir and swell at 25 degrees Celsius for 25 hours, then stir and react at 77.5 degrees Celsius for 4.5 hours, and finally mature at 87.5 degrees Celsius The reaction was stirred for 10.5 hours at 100°C to obtain ethylstyrene-divinylbenzene polymer microspheres with a crosslinking degree of 70%.
[0031] The 3 difference from Example 1 is that the catalyst is anhydrous zinc chloride; the chloromethylation reaction temperature is 50 degrees Celsius with the chloromethylation reagent paroxymethylene and concentrated hydrochloric acid solution, and the...
Embodiment 3
[0036] 1 difference with embodiment one is that the protective colloid reagent is light calcium carbonate, 500 milliliters of deionized water, add 10 grams of protective colloid reagent light calcium carbonate, the divinylbenzene of content 85% and initiator are n-octanoyl peroxide .
[0037] The difference between 2 and Example 1 is that the light calcium carbonate solution dissolved in 1 is added to the three-necked bottle, under nitrogen atmosphere, first stir and swell at 35 degrees Celsius for 23 hours, then stir and react at 82.5 degrees Celsius for 3.5 hours, and finally Aging was carried out at 92.5 degrees Celsius for 9.5 hours with stirring to prepare ethylstyrene-divinylbenzene polymer microspheres with a crosslinking degree of 85%.
[0038] The 3 difference from Example 1 is that the catalyst is anhydrous ferric chloride, and the chloromethylation reaction temperature is 70 degrees Celsius with the chloromethylation reagent paroxymethylene and concentrated hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com