Aromatic heterocyclic amide substituted diarylurea compound, preparation method and application thereof
A technology of heterocyclic amide and diaryl urea, which is applied in the field of biomedicine, can solve the problems that chemical drugs cannot achieve therapeutic effects, hair loss, etc., achieve good application prospects and scientific research value, inhibit proliferation and migration, and achieve the effect of cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
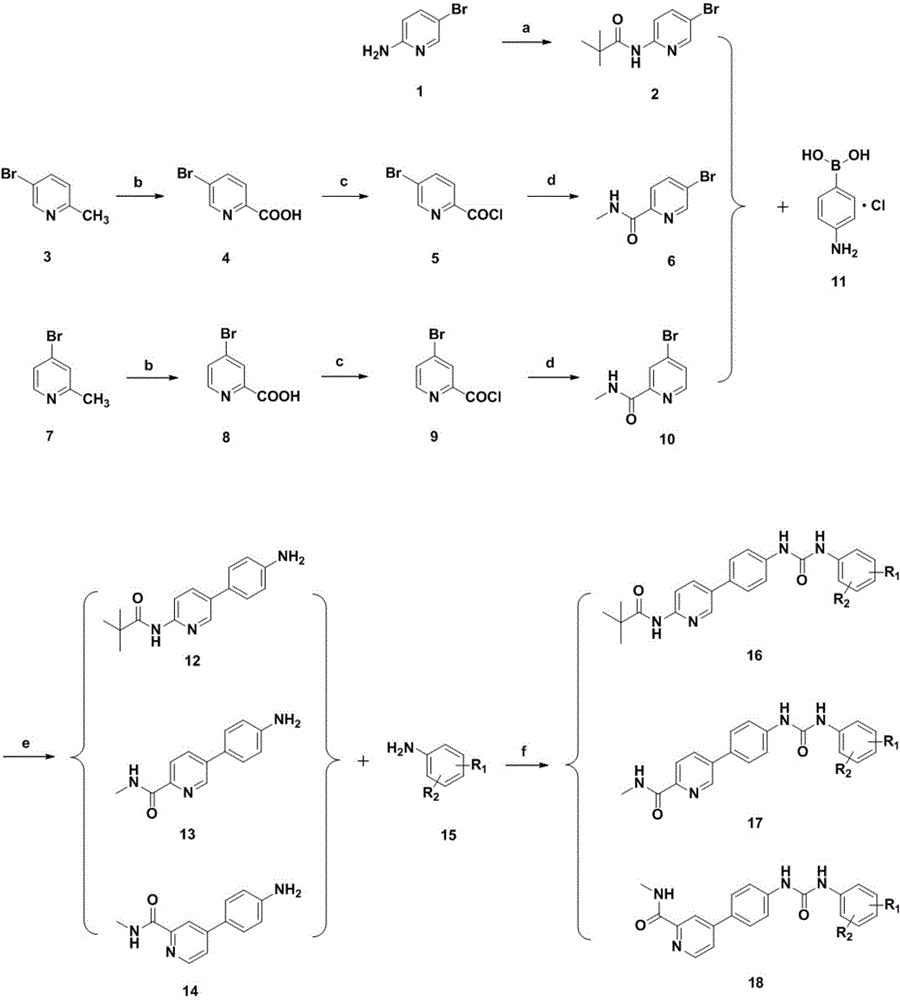
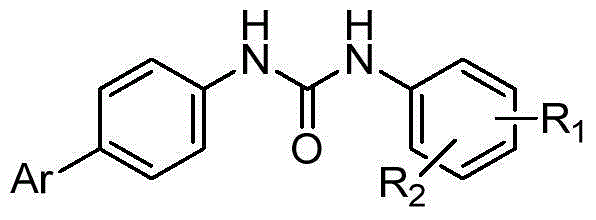
[0041] In the structural formula of the compound, Ar is a pyridine heterocycle containing a pivalylamino group, R 1 is trifluoromethyl, R 2 For bromine, prepared by the following steps (see figure 1 ):
[0042] 1) Preparation of N-(5-bromopyridin-2-yl)-2,2-dimethylpropionamide (compound 2) from 5-bromo-2-aminopyridine (compound 1)
[0043]Dissolve 8.6g of 5-bromo-2-aminopyridine (compound 1) in anhydrous dichloromethane, add 25ml of anhydrous triethylamine, stir in ice bath for 30min, then slowly add 12ml of pivaloyl chloride in anhydrous Dichloromethane solution, after the dropwise addition, react at room temperature overnight. Extract the reaction solution with dichloromethane for 2 to 3 times, then wash the extract with water for 2 times, and finally wash with saturated sodium bicarbonate solution and saturated brine successively, dry over anhydrous sodium sulfate, spin dry to obtain a residue, and use chromatography Column separation obtained 7.3 g of solid N-(5-bromop...
Embodiment 2
[0055] In the structural formula of the compound, Ar is a pyridine heterocycle containing a pivalylamino group, R 1 is trifluoromethoxy, R 2 for bromine.
[0056] Steps 1) to 2) are the same as steps 1) to 2) in Example 1, that is, N-(5-bromopyridin-2-yl)-2 is prepared from 5-bromo-2-aminopyridine (compound 1), 2-Dimethylpropanamide (compound 2), and then prepared N-[5-(4-aminophenyl)pyridin-2-yl] by Suzuki coupling reaction with p-aminophenyl borate hydrochloride (compound 11) -2,2-Dimethylpropanamide (compound 12).
[0057] 3) From N-[5-(4-aminophenyl)pyridin-2-yl]-2,2-dimethylpropionamide (compound 12) and 4-bromo-2-trifluoromethoxyaniline (compound 15) N-(5-(4-(3-(4-bromo-2-(trifluoromethoxy) phenyl) ureido) phenyl) pyridin-2-yl) pivalamide ( Compound 16), the specific operation steps are:
[0058] Under ice bath conditions, dissolve 0.13g (0.44mmol) bis(trichloromethyl)carbonate (BTC) with 15mL redistilled dichloromethane and stir for 5min, then slowly add 0.28g (1.1...
Embodiment 3
[0064] In the structural formula of the compound, Ar is a pyridine heterocycle containing a pivalylamino group, R 1 , R 2 for chlorine.
[0065] Steps 1) to 2) are the same as steps 1) to 2) in Example 1, that is, N-(5-bromopyridin-2-yl)-2 is prepared from 5-bromo-2-aminopyridine (compound 1), 2-Dimethylpropanamide (compound 2), and then prepared N-[5-(4-aminophenyl)pyridin-2-yl] by Suzuki coupling reaction with p-aminophenyl borate hydrochloride (compound 11) -2,2-Dimethylpropanamide (compound 12).
[0066] 3) By condensation reaction of N-[5-(4-aminophenyl)pyridin-2-yl]-2,2-dimethylpropionamide (compound 12) and 3,4-dichloroaniline (compound 15) To prepare N-(5-(4-(3-(3,4-dichlorophenyl)ureido)phenyl)pyridin-2-yl)pivalamide (compound 16), the specific operation steps are:
[0067] Under ice-bath conditions, dissolve 0.18g (0.6mmol) of bis(trichloromethyl)carbonate (BTC) with 15mL of redistilled dichloromethane and stir for 5min, then slowly add 0.24g (1.5mmol) of 3,4 -T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com