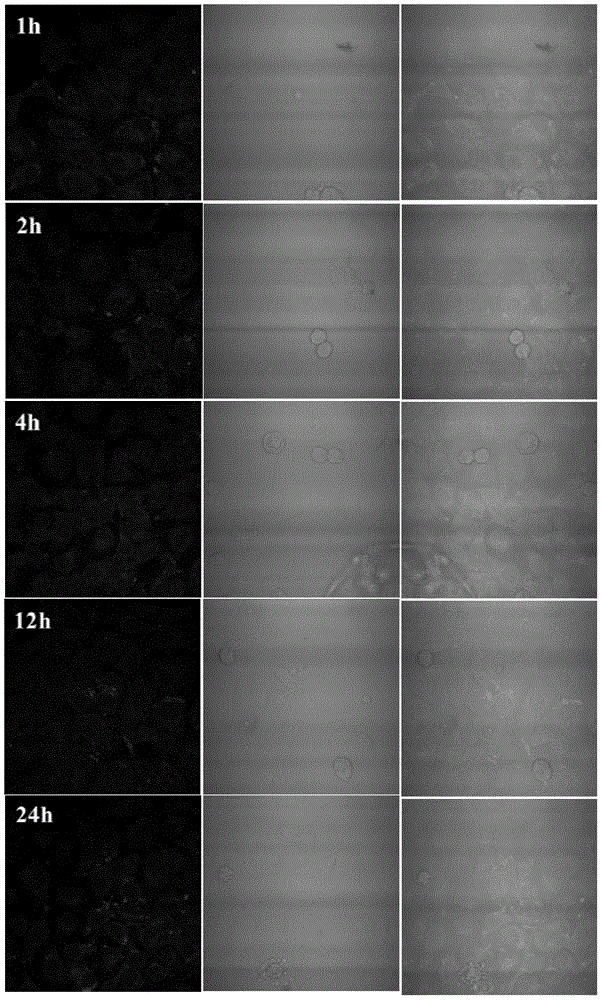
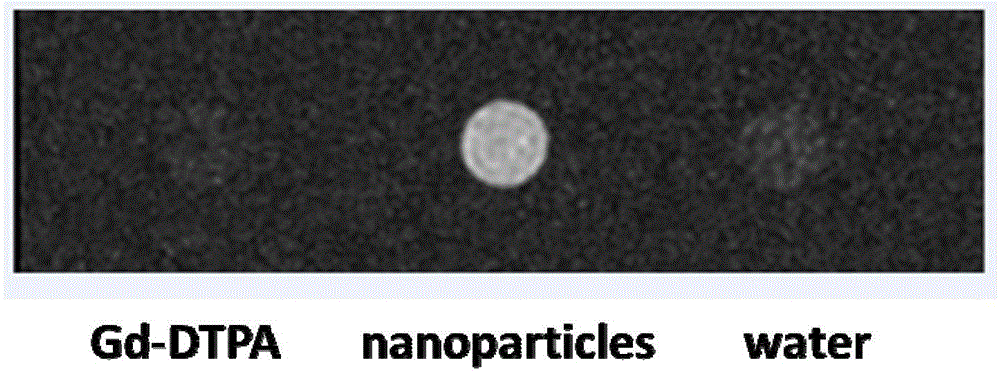
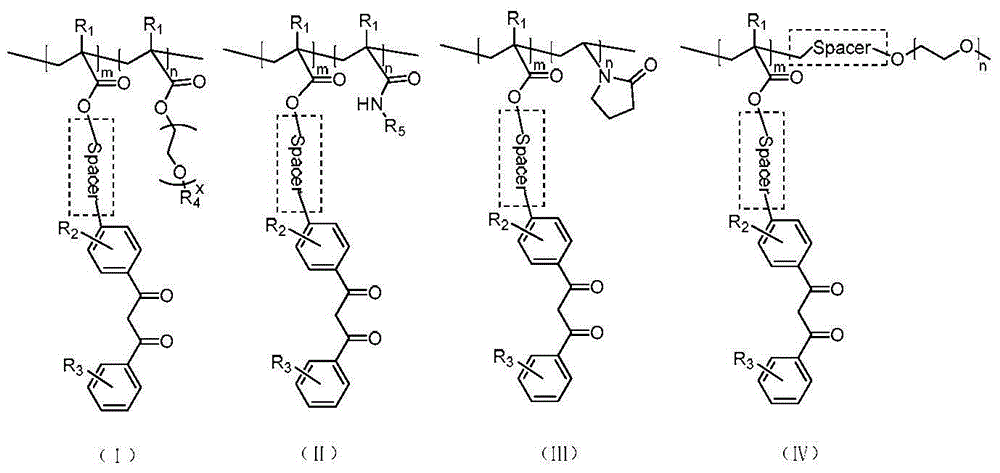
Amphiphilic segmented copolymers, nanoparticles containing amphiphilic segmented copolymers, preparation method of amphiphilic segmented copolymers and use of nanoparticles
A technology of amphiphilic blocks and nanoparticles, which is applied in biochemical equipment and methods, microbial measurement/inspection, preparations for in vivo tests, etc., can solve the problems of short residence time and achieve narrow emission peaks and low emission peaks. Effect of cytotoxicity and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] 1.41g of 4-(3-oxo-3-phenylpropionyl)phenyl methacrylate (DKMA) (CAS: 157174-85-1), 4.15mg of chain transfer agent dithiobenzoic acid-2- Add phenylpropan-2-ester (CDB) (CAS:201611-77-0), 0.826mg AIBN, 10mL anisole into the reaction flask, the molar ratio of DKMA, AIBN and CDB is 300:0.33:1, 70℃ The reaction was carried out for 12 hours, dissolved and precipitated in 100 mL of ether for 3 times to remove small molecular monomers, and vacuum-dried to obtain polymethacrylic acid-4-(3-oxo-3-phenylpropionyl)phenyl ester (PDKMA). The yield was 50%, NMR calculated degree of polymerization is 152, SEC number average molecular weight is 33.8kDa, molecular weight distribution is 1.28.
[0063] Add 1.810 g of oligoethylene glycol methacrylate (OEGMA), 0.564 mg of PDKMA, 1.519 mg of AIBN and 5 mL of anisole into the reaction flask, the molar ratio of PDKMA, AIBN and OEGMA is 1:0.33:130, 60 °C After the reaction was completed, 200 mL of ether was precipitated three times to remove t...
Embodiment 2
[0072] 1.2g of 4-(3-oxo-3-phenylpropionyl)phenyl acrylate (DKA), 5.55mg of chain transfer agent-2-phenylpropan-2-dithiobenzoate (CDB), Add 0.744mg AIBN and 5mL anisole into the reaction flask, the molar ratio of DKA, AIBN and CDB is 200:0.33:1, react at 70°C for 24 hours, dissolve and precipitate in 200mL ether three times to remove small molecular monomers, and dry in vacuum Obtain polyacrylic acid-4-(3-oxo-3-phenylpropionyl)phenyl ester (PDKA), the yield is 20%, the NMR calculation degree of polymerization is 51, the SEC number average molecular weight is 15.8kDa, and the molecular weight distribution is 1.30 .
[0073] Add 0.8 g oligoethylene glycol methacrylate (OEGMA), 0.24 g PDKA, 0.866 mg AIBN and 5 mL anisole into the reaction flask, the molar ratio of PDKA, AIBN and OEGMA is 1:0.33:100, 60 °C After the reaction was completed, 200 mL of ether was precipitated three times to remove the monomer, and vacuum-dried to obtain polyacrylic acid-4-(3-oxo-3-phenylpropionyl)phen...
Embodiment 3
[0075] 1.45g of 4-(3-oxo-3-phenylpropionyl)phenyl methacrylate (DKMA), 4.15mg of chain transfer agent-2-phenylpropan-2-dithiobenzoate (CDB ), 0.826mg AIBN, and 10mL anisole were added to the reaction flask, the molar ratio of DKMA, AIBN and CDB was 310:0.33:1, reacted at 70°C for 12 hours, dissolved and precipitated in 300mL ether three times to remove small molecular monomers, Vacuum-dried to obtain polymethacrylic acid-4-(3-oxo-3-phenylpropionyl)phenyl ester (PDKMA), the yield was 53%, the nuclear magnetic calculation degree of polymerization was 168, and the SEC number average molecular weight was 34.8kDa, The molecular weight distribution was 1.28.
[0076] Add 0.52g N-isopropylacrylamide (NIPAM), 0.373g PDKMA, 1.519mg AIBN and 5mL anisole into the reaction flask, the molar ratio of PDKMA, AIBN and NIPAM is 1:0.33:728, react at 60°C 8h, after the reaction, 200mL ether precipitated 3 times to remove the monomer, and vacuum dried to obtain polymethacrylic acid-4-(3-oxo-3-ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com