Preparation method of mono-dispersed gelatin embolic microsphere with precisely-controlled particle size
A monodisperse, gelatin technology, used in the preparation of microspheres, microcapsule preparations, inactive components of polymer compounds, etc., can solve the residual product quality of cross-linking curing agent, the troublesome post-processing of curing methods, and the wide distribution of grade particle size, etc. To improve the clinical embolization effect, reduce the probability of false embolization, and achieve uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
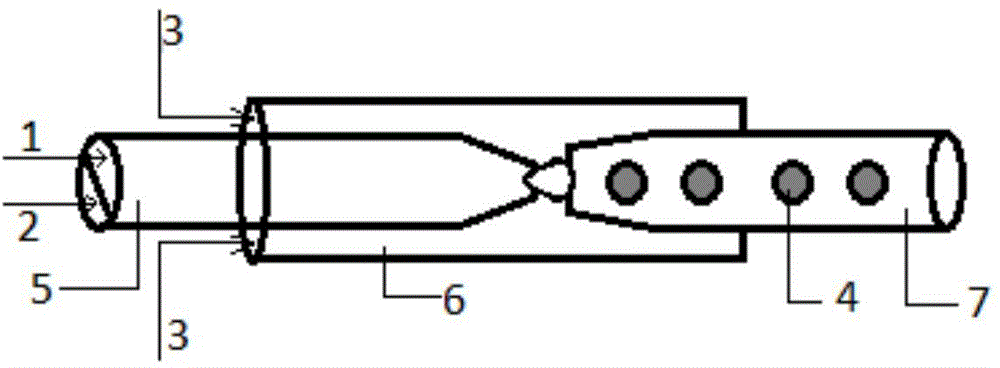
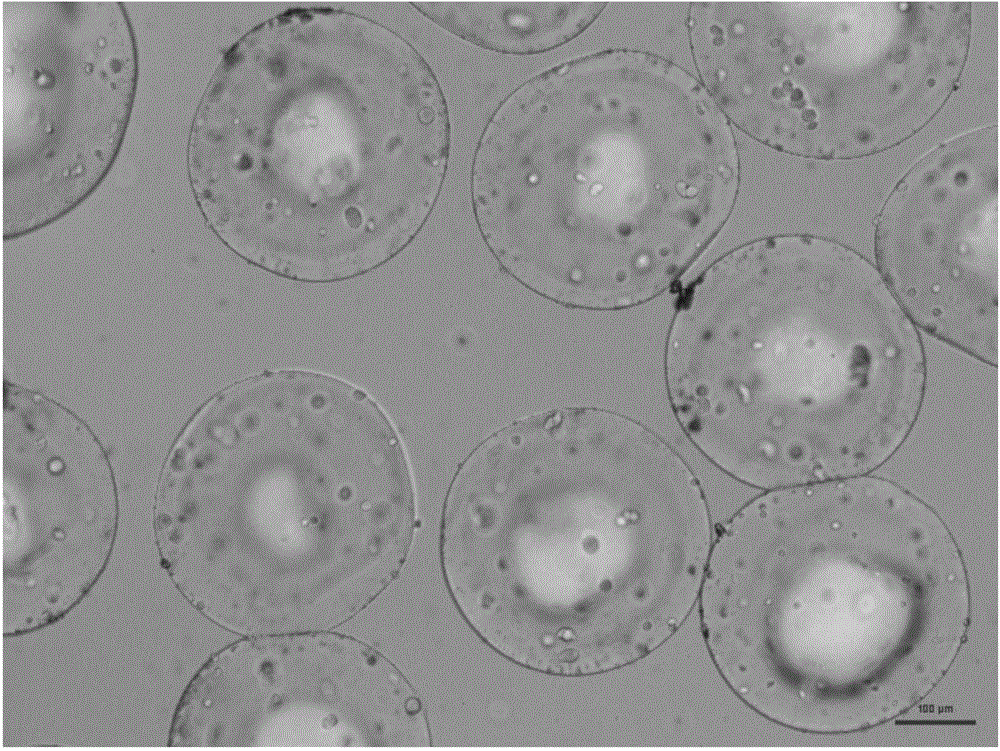
[0045] Preparation mass percent concentration is 2% gelatin aqueous solution and 2% tannic acid aqueous solution as dispersed phase, preparation contains the liquid paraffin solution that mass percent concentration is 1% Span80 as continuous phase, three kinds of solutions (gelatin aqueous solution, tannic acid solution) aqueous solution, liquid paraffin solution containing Span80) into syringes respectively, and placed on three micro-injection pumps respectively, after connecting the microchannel reactor, adjust the flow of gelatin aqueous solution, tannic acid aqueous solution and liquid paraffin solution containing Span80 0.3mL / h, 0.5mL / h, and 7mL / h, respectively, to obtain W / O droplets with a particle size of 580±5μm. Collect the generated gelatin droplets and place them in an oven at 30°C for 30 minutes to solidify. The solidified gelatin microspheres sink to the bottom of the beaker, pour to remove the upper layer of liquid paraffin, add pure water, ultrasonically break t...
Embodiment 2
[0049] Preparation mass percentage concentration is 10% gelatin aqueous solution and 15% glutamine transaminase aqueous solution as dispersed phase, the preparation contains the dodecane solution that mass percentage concentration is 2% Span80 as continuous phase, three kinds of solutions are packed in the syringe respectively , and placed on three micro-injection pumps respectively, after connecting the microchannel reactor, adjust the flow rates of the gelatin aqueous solution, the transglutaminase aqueous solution and the dodecane solution containing Span80 to be 0.3mL / h and 0.5mL / h respectively, 7mL / h to obtain W / O droplets with a particle size of 510±5um; collect the generated gelatin droplets and place them in an oven at 40°C to cure for 90min. Dioxane solution, supplemented with pure water, ultrasonic demulsification, centrifugation until the liquid is clear after ultrasonication, the particle size of gelatin microspheres is 290±5μm, and after freeze-drying, the particle...
Embodiment 3
[0053] The preparation mass percentage concentration is 2% gelatin aqueous solution and 5% genipin aqueous solution as dispersed phase, preparation contains the liquid paraffin solution that mass percentage concentration is 2% EM90 as continuous phase, three kinds of solutions are packed in the syringe respectively, and Placed on three micro-injection pumps respectively, after connecting the microchannel reactor, adjust the flow rate of gelatin aqueous solution, genipin aqueous solution and liquid paraffin solution containing EM90 to 0.5mL / h, 0.7mL / h, 10mL / h respectively, W / O droplets with a particle size of 650±5um were obtained. Collect the generated gelatin droplets and place them in an oven at 30°C for 120 minutes to solidify. The solidified gelatin microspheres sink to the bottom of the beaker, pour to remove the upper layer of liquid paraffin, add pure water, ultrasonically break the emulsion, and centrifuge until the liquid is clear after ultrasonication. , the particle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com