Preparation method of zidovudine and intermediate thereof
A technology of zidovudine and intermediates, applied in the field of pharmaceutical chemical synthesis, can solve the problems of high reaction temperature, high cost of raw materials, cumbersome operation process, etc., to reduce the azidation reaction temperature, facilitate process production, and solvent recovery rate high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] The present invention will be described below in conjunction with specific embodiments. The given embodiments are only general examples of the products or methods of the present invention, which help to better understand the present invention, but do not limit the scope of the present invention.
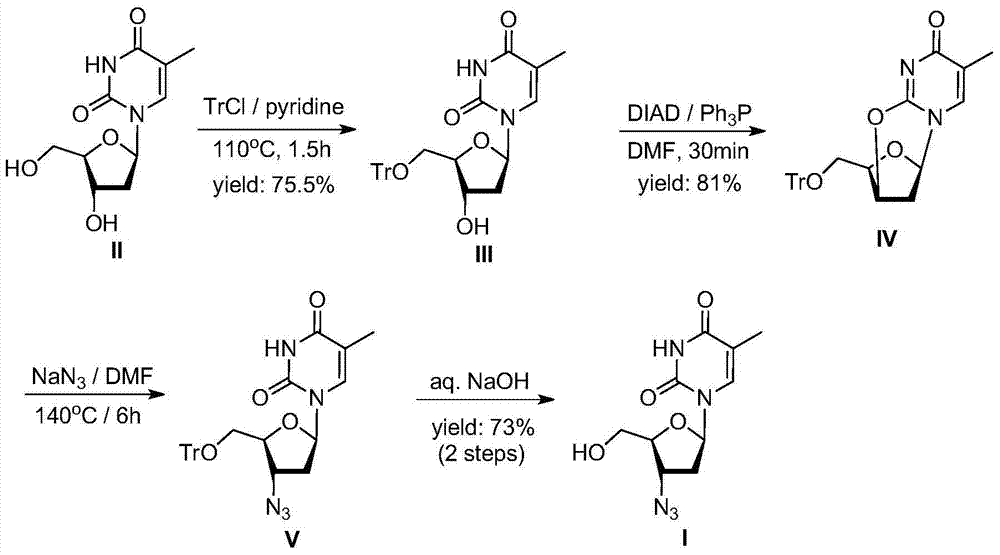
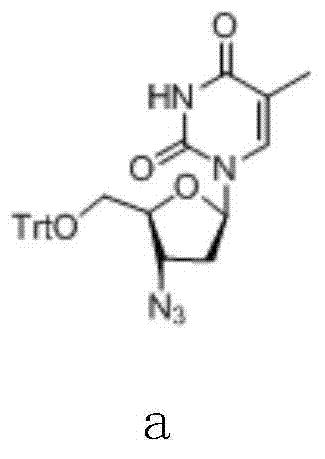
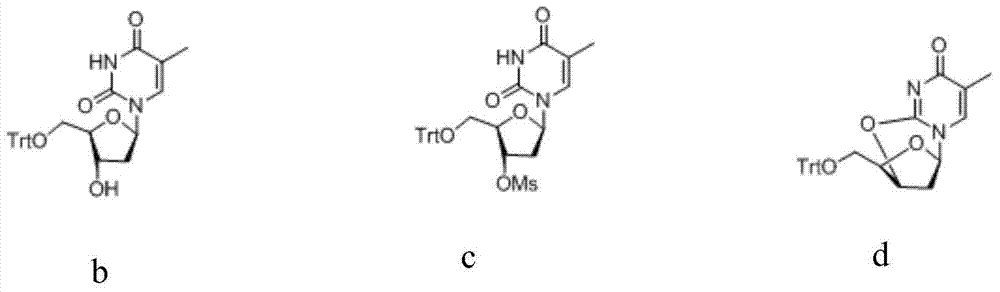
[0039] Synthesis of Zidovudine Oxygen Bridge Compound (d)
[0040] Add β-thymidine (f, 100.0 g, 0.41 mol) and pyridine (400 mL) into the reaction flask, stir, and slowly raise the temperature to 30-60° C. to dissolve. After complete dissolution, triphenylchloromethane (125.5 g, 1.1 eq) was added, and the reaction was maintained at 30-60° C. for 16 hours, and the end point was controlled by TLC. After confirming the completion of the reaction, cool down and slowly add methanesulfonyl chloride (49.3g, 1.05eq) dropwise under stirring at 0-5°C. After the dropwise addition, it is naturally raised to room temperature and reacted for 2-8 hours, and the end point is controlled by TLC....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com