Difluoro monomer containing tetraphenylethylene groups and application of difluoro monomer for preparing polyaryletherketone polymers
A technology of tetrastyrene and polyaryletherketone is applied in the field of polymer materials and their preparation, and can solve the problem of no fluorescence and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
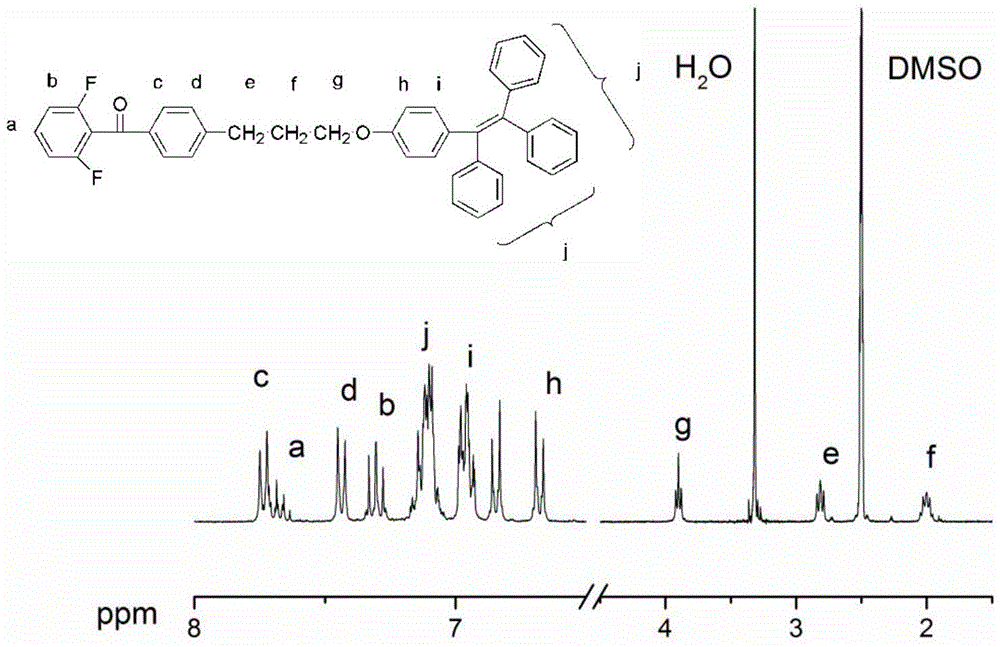
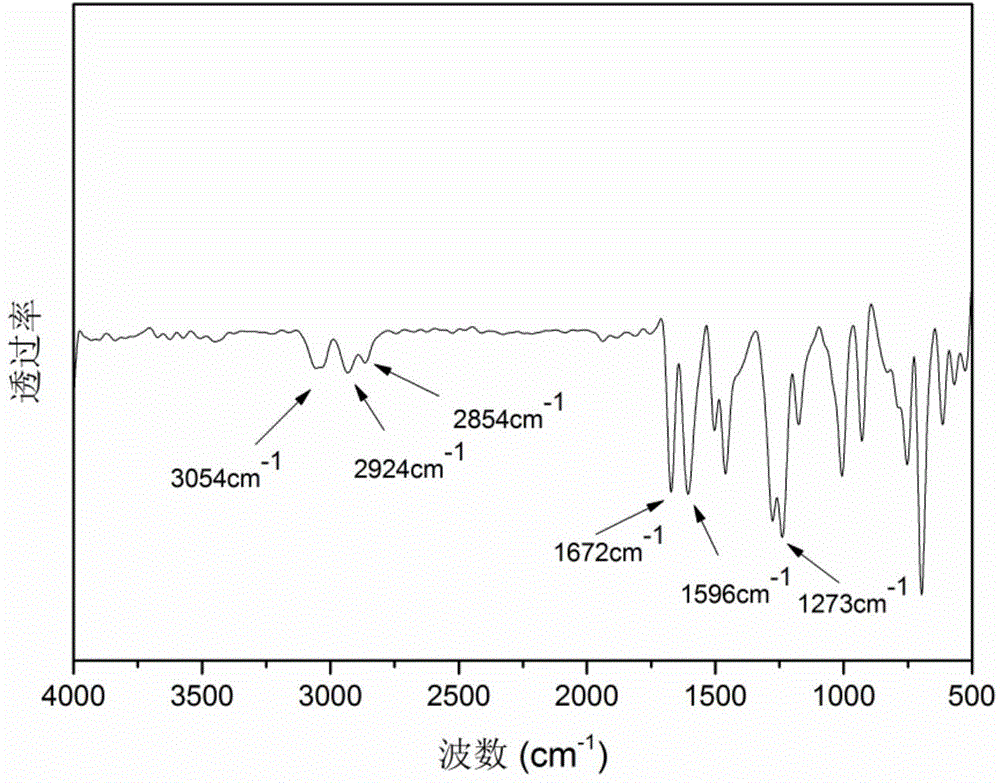
[0037] The first step reaction: under the conditions of ice-water bath and argon protection, add 90ml methylene chloride and 14.6g (0.11mol) anhydrous aluminum trichloride to the 250ml three-necked flask equipped with mechanical stirring and constant pressure dropping funnel, pass 17.6g (0.1mol) 2,6-difluorobenzoyl chloride and 15.7g (0.1mol) 3-phenylpropyl bromide were successively dropped into the three-necked flask under stirring through a constant pressure dropping funnel, and the dripping was completed in 30 minutes, and the Water bath reaction for 8 hours. After the reaction system is down to room temperature, the material is discharged in a 10% hydrochloric acid aqueous solution, and the organic phase is washed 4 times successively with a 5% aqueous sodium hydroxide solution and distilled water for liquid separation, and the obtained organic phase is washed with anhydrous Magnesium sulfate was dried, filtered, and then the solvent was distilled off under reduced pressur...
Embodiment 2
[0041] The first step reaction: under the protection condition of ice-water bath argon, add 180ml dichloromethane and 29.2g (0.22mol) anhydrous aluminum trichloride to the 250ml three-neck flask equipped with mechanical stirring and constant pressure dropping funnel, through constant 35.2 g (0.2 mol) of 2,6-difluorobenzoyl chloride and 31.4 g (0.2 mol) of 3-phenylpropyl bromide were sequentially dripped into the three-necked flask under stirring through the pressure dropping funnel, and the dripping was completed in 30 minutes, and the mixture was cooled in an ice-water bath. React for 8 hours. After the reaction system is down to room temperature, the material is discharged in a 10% hydrochloric acid aqueous solution, and the organic phase is washed 4 times successively with a 5% aqueous sodium hydroxide solution and distilled water for liquid separation, and the obtained organic phase is washed with anhydrous Dry over magnesium sulfate, filter, then distill off the solvent u...
Embodiment 3
[0045] (1) The preparation of difluoro monomers containing tetraphenylethylene groups is the same as described in Example 1.
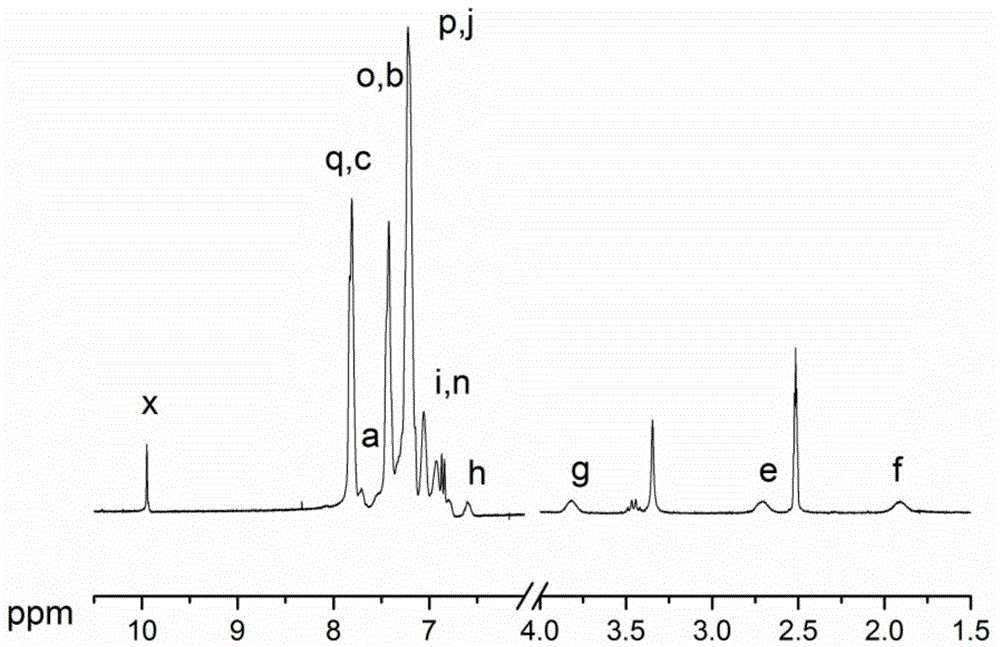
[0046] (2) Add (2,6-difluorophenyl) (4-(3-(4-1, 2,2-triphenylvinyl)phenoxy)propyl)phenyl)methanone 0.24268g (0.4mmol), 2,2'-di(4-hydroxyphenyl)propane 0.93146g (4.08mmol), 0.7855g (3.6mmol) of 4,4'-difluorobenzophenone and 0.6634g (4.8mmol) of anhydrous potassium carbonate were dissolved in sulfolane (8.3ml), water-carrying agent toluene (5ml) was added, and nitrogen gas was introduced for 15 Minutes until all the monomers are dissolved, heat up to 130°C and reflux for 2.5 hours, then release the water-carrying agent toluene and the water generated by the reaction, raise the temperature of the reaction system to 180°C, and complete the copolymerization reaction after 8 hours. The viscous solution was slowly poured into 10% hydrochloric acid ice water solution by mass fraction to obtain flexible polymer thin strips, which were crushed into powder with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com