Method for compounding BNC 105
A solvent and reaction temperature technology, applied in the field of preparation of BNC105, can solve the problems of increased energy consumption, unsuitable for industrial production, long synthesis route, etc., and achieves the effects of mild reaction conditions, simple and feasible operation, and cheap and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 prepares the method for BNC105
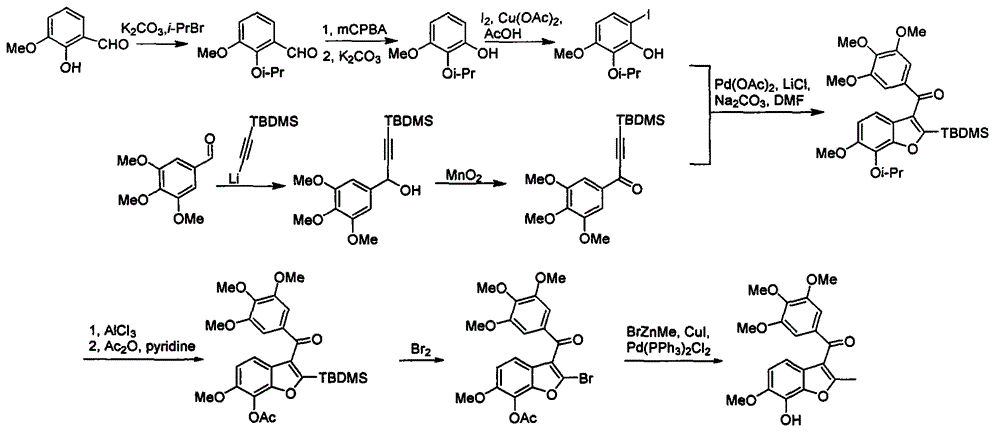
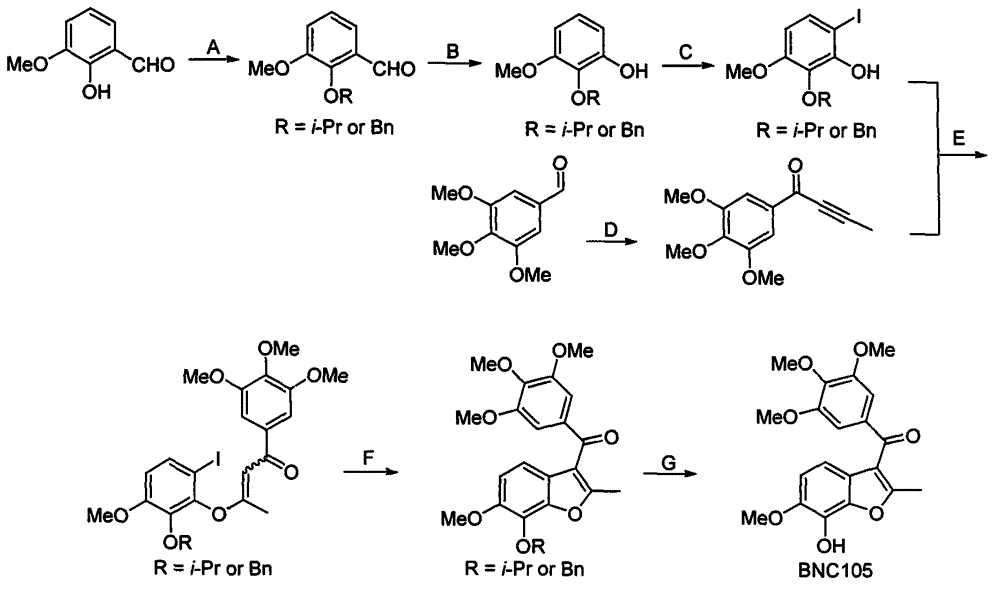
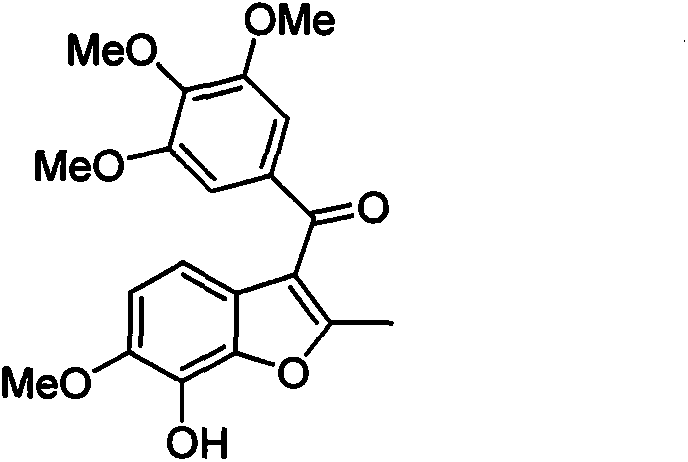
[0029] A kind of preparation method of BNC105, see figure 2 , using o-vanillin and 3,4,5-trimethoxybenzaldehyde as starting materials, respectively through hydroxyl protection, aldehyde group oxidation hydrolysis to phenol, iodide to generate o-iodophenol intermediates and nucleophilic addition, oxidation The method of producing acetylene ketone intermediate; acetylene ketone and o-iodophenol intermediate is subjected to Michael addition, free radical ring closure and deprotection to obtain the product BNC105.
[0030] Concrete preparation steps are as follows:
[0031] A: o-vanillin and 2-bromopropane or benzyl bromide react with a base and a phase transfer catalyst at room temperature and in a specific solvent to obtain 2-isopropoxy-3-methoxybenzaldehyde or 2-benzyloxy-3 -Methoxybenzaldehyde. The base used in the reaction is sodium tert-butoxide, potassium tert-butoxide, sodium hydride, potassium hydride, calcium hydri...
Embodiment 2
[0038] The specific preparation steps of embodiment 2 BNC105
[0039] (1) Synthesis of 2-isopropoxy-3-methoxybenzaldehyde
[0040] Weigh o-vanillin (500g, 3.286mol, 1.0eq), potassium carbonate (908g, 6.572mol, 2.0eq) and dissolve in 2L acetone, add tetrabutylammonium bromide (54g, 0.165mol, 0.05eq), Stir at room temperature for 15 minutes, drop in 2-bromopropane (405mL, 4.272mol, 1.3eq), dropwise, stir at room temperature for 24 hours, filter, concentrate, dissolve the residue in ethyl acetate, wash with water three times, distill under reduced pressure, and dry in vacuo to obtain 612 g of light yellow oily liquid product, HPLC content 98%, yield 96%.
[0041] 1 H NMR (500MHz, CDCl 3 )δ10.45(d, J=0.7Hz, 1H), 7.41(dd, J=7.3, 2.1Hz, 1H), 7.16-7.04(m, 2H), 4.62(dt, J=12.3, 6.2Hz, 1H ), 3.87(s, 3H), 1.32(d, J=6.2Hz, 6H); 13 C NMR (125MHz, CDCl 3 )δ191.0, 153.4, 150.7, 131.0, 123.8, 119.1, 118.0, 76.4, 56.1, 22.4; ESI-MS: [M+H] + 195.1[M+Na] + 217.2.
[0042] (2) Synthesis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com